Long-term outcomes of liver transplantation using grafts from donors with active hepatitis B virus replication: a multicenter cohort study
YoungRok Choi1,2, Sujin Kang1,2, Boram Lee1,3, Kyung Chul Yoon1,4, Sola Lee1,2, Sanggyun Suh1,2, Su young Hong1,2, Eui Soo Han1,2, Jeong-moo Lee1,2, Suk Kyun Hong1,2, Hae Won Lee1,3, Kwang-Woong Lee1,3, Nam-Joon Yi1,2, Kyung-Suk Suh1,2, Jai Young Cho1,2.
1Surgery, Seoul National University College of Medicine, Seoul, Korea; 2Surgery, Seoul National University Hospital, Seoul , Korea; 3Surgery, Seoul National University Bundang Hospital, Seongnam, Korea; 4Surgery, Seoul National University Boramae Medical Center, Seoul, Korea
Background: Liver grafts from donors with hepatitis B infection contributed to expanding the donor pool under the Hepatitis B immunoglobulin (HBIG) and antiviral agents (NA: Nucleos(t)ide analogs) in the hepatitis B virus (HBV) endemic area. The purpose of this study is to describe the long-term outcome of liver transplantation (LT) using grafts from donors with active and chronic hepatitis B virus infection.
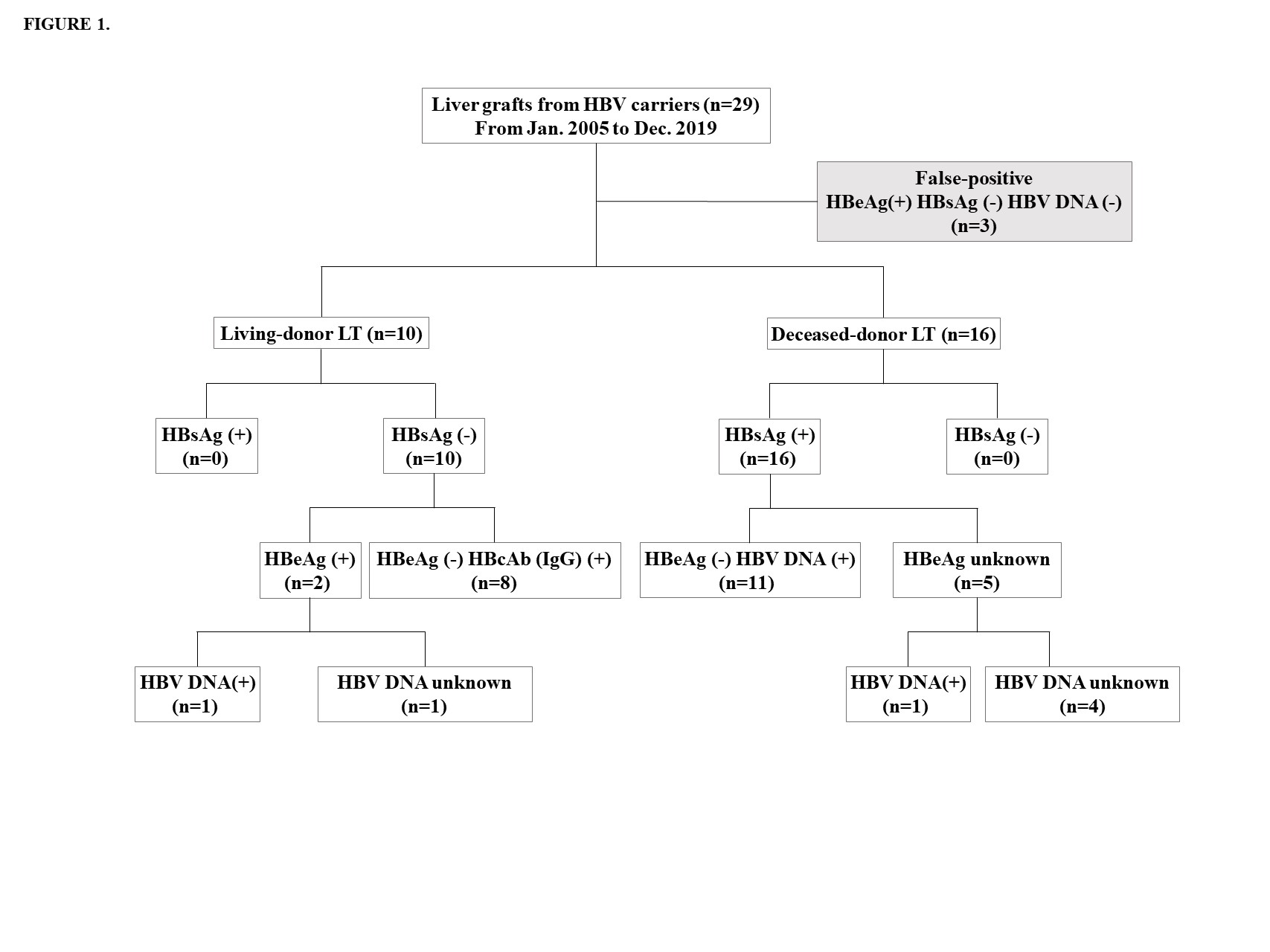
Methods: Between January 2000 and April 2019, 2260 liver transplants (LTs) were performed at Seoul National University (SNU) Hospital, SNU Bundang Hospital (SNUBH), and Seoul Metropolitan Government-SNU Boramae Hospital. Twenty-six (1.2%) grafts from donors with HBsAg (+), HBeAb (+), or HBV DNA (+) were classified as active and chronic HBV hepatitis grafts and retrospectively reviewed. The demographics of donors and recipients, as well as the outcomes of transplantation, were analyzed. HBV reactivation has been defined as an increase in viral DNA in HBsAg (+) grafts and seroconversion to HBsAg positive grafts in chronic hepatitis grafts. Additionally, we used the chronic HBV infection stage to evaluate and manage recipients who received HBV-infected grafts.
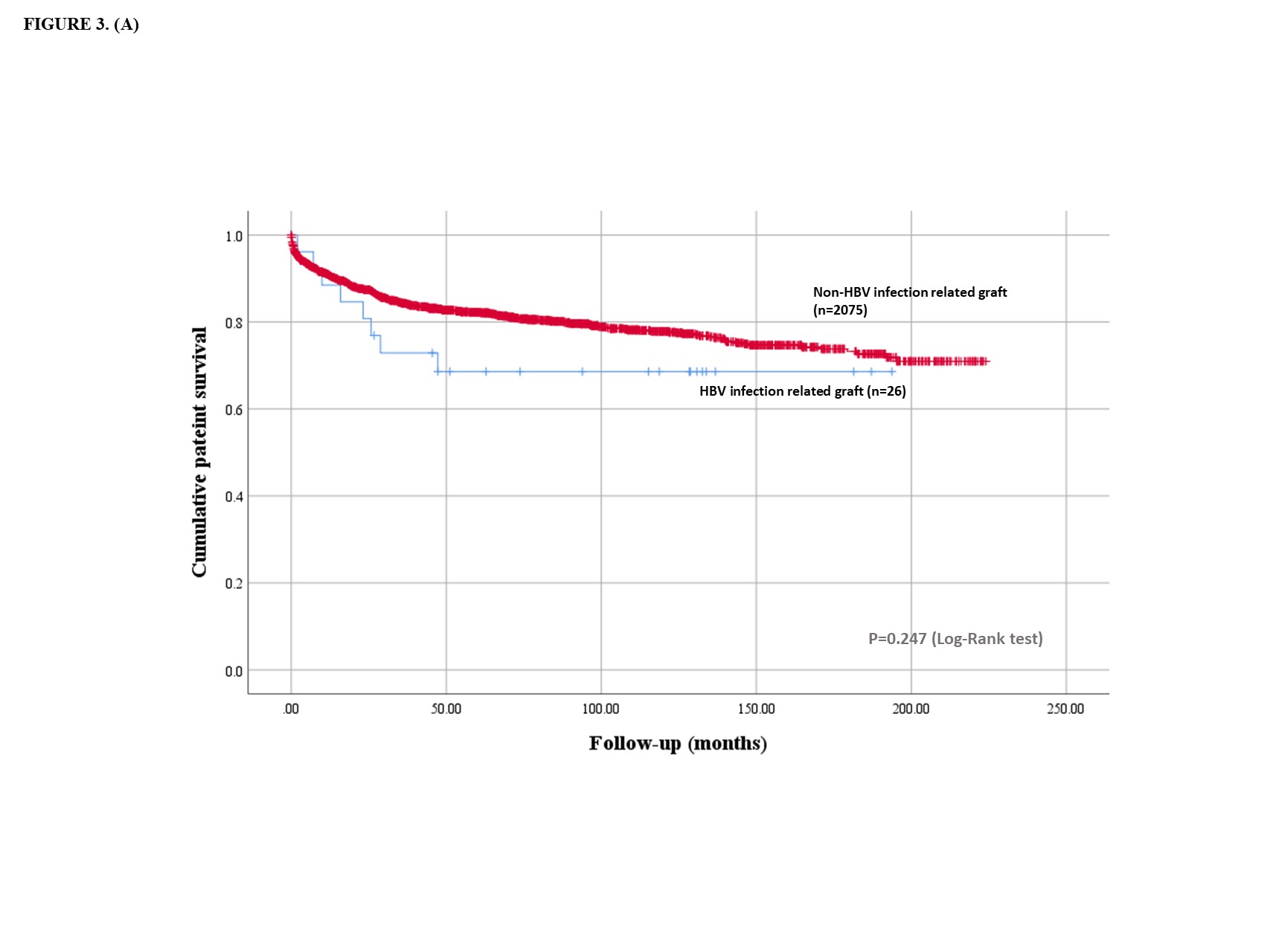
Results: Sixteen LT were performed on deceased donors using active HBsAg (+) grafts. Ten living donor LT were performed using inactive HBV grafts; eight patients with inactive hepatitis; HBsAg (-), HBcAb (+), and HBV DNA (+), and two patients with chronic HBV hepatitis with seroconversion; HBsAg (-), HBsAb (+), and HBeAg (+) (Figure 1). The recipients were aged 59.0 ± 10.3 years and had a MELD score of 19.9 ± 8.4. The mean duration of follow-up was 82.6 ± 60.1 months. Depending on the donor and recipient's serology, NA and HBIG were administered during the perioperative period. Deaths (n=8) occurred between 2.0 and 47.3 months following LT. All deaths were reported in DDLT. When compared with LT using grafts without HBV, LT using HBV infected grafts did not show any significant difference in patient survival (30.8% vs. 18.6%, p=0.247) (Figure 2). The most common causes of death were infection (n=4) and HCC recurrence (n=3). All recurred HCC patients died of cancer. HBV reactivation was identified in 1 patient but resolved spontaneously without additional management. All 10 LDLT recipients survived and were in good condition during follow-up. Survivors were in inactive or resolved status for HBV infection under the HBIG and NA. No graft failure was observed. Fourteen patients followed-up more than 5 years were stable and no increase in HCC recurrence rate was observed 5 years after transplantation.
Conclusion: Considering their long-term outcomes, liver grafts with active and chronic HBV infection can be safely used for LT in HBV endemic area.

right-click to download
