Proton-pump inhibitor use, fatigue and health-related quality of life in kidney transplant recipients
Tim Knobbe1, Daan Kremer1, Rianne M Douwes1, Michele F Eisenga1, António W Gomes-Neto1, Coby Annema2, J Casper Swarte1, Frank Klont3,4, Gerjan Navis1, Stefan P Berger1, Stephan JL Bakker1.
1Department of Internal Medicine, Division of Nephrology, University Medical Center Groningen, University of Groningen, Groningen, Netherlands; 2Department of Health Sciences, Section of Nursing Research, University Medical Center Groningen, University of Groningen, Groningen, Netherlands; 3Unit of PharmacoTherapy, -Epidemiology & -Economics, Groningen Research Institute of Pharmacy, University of Groningen, Groningen, Netherlands; 4Department of Clinical Pharmacy and Pharmacology, University Medical Center Groningen, University of Groningen, Groningen, Netherlands
TransplantLines Investigators.
Background: The role of the gut-brain interaction in fatigue etiology and health-related quality of life (HRQoL) has become increasingly clear in the past decades. Recently, it has been shown that the gut microbiota can be affected by the use of proton-pump inhibitors (PPIs). Given the common use of PPIs among kidney transplant recipients (KTR), we hypothesized that PPI use may be an important and underappreciated determinant of fatigue and HRQoL in this population.
Methods: Data of KTR (≥1 year after transplantation) from the TransplantLines Biobank and Cohort Study were used. PPI use was retrieved from medical records and verified with study participants. Severe fatigue and HRQoL were assessed using the validated CIS20R and SF-36 questionnaires.
Results: A total of 937 KTR (39% male, mean age 56±13 years) were included at a median of 3 [1-10] years after transplantation. PPIs were used by 656 (70%) KTR. Severe fatigue was more prevalent among PPI users compared to non-PPI users (36% vs. 22%, p<0.001). In addition, both physical and mental HRQoL scores were lower among PPI users compared to non-PPI users (physical: 67 ± 22 vs. 75 ± 19, p<0.001; mental: 75 ± 18 vs. 79 ± 17, p<0.001). In regression analyses, PPI use was independently associated with a higher risk of severe fatigue (OR 2.25, 95%CI 1.53 to 3.30, p<0.001), lower physical HRQoL (B -6.42, 95%CI -9.48 to -3.36, p<0.001) and lower mental HRQoL (B -4.10, 95%CI -6.78 to -1.43, p=0.003). Additional analyses showed that these associations were dose-dependent, present among all individually assessed PPI types and dependent on duration of PPI use.
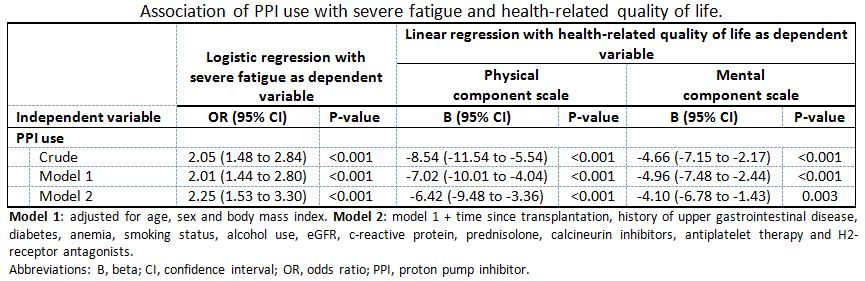
Conclusion: PPI use is associated with a more than two times higher risk of severe fatigue, and with lower physical and mental HRQoL among KTR. These associations were dose-dependent, consistent for all individually assessed PPI types, and dependent on the duration of PPI use. This raises the hypothesis that critical evaluation of PPI use may be an easy target to alleviate fatigue and improve HRQoL in this population.
The TransplantLines Biobank and Cohort study was supported by a grant from Astellas BV and Chiesi Pharmaceuticals BV.

right-click to download
