Effect of vitamin K2-supplementation on calcification propensity and vascular stiffness in vitamin K-deficient kidney transplant recipients: a randomized, double-blind, placebo-controlled clinical trial
Daan Kremer1, Coby Eelderink1, Ineke J Riphagen2, Tim J Knobbe1, Leon J Schurgers3, Andreas Pasch4, Stephan JL Bakker1, Martin H de Borst1, Charlotte A te Velde-Keyzer1.
1Department of Internal Medicine, Division of Nephrology, University of Groningen and University Medical Center Groningen, Groningen, Netherlands; 2Department of Laboratory Medicine, University of Groningen and University Medical Center Groningen, Groningen, Netherlands; 3Department of Biochemistry, Cardiovascular Research Institute Maastricht (CARIM), University of Maastricht, Maastricht, Netherlands; 4Department of Clinical Chemistry, University Hospital Bern (Inselspital), Bern, Switzerland
Introduction: Even after successful transplantation, kidney transplant recipients (KTR) remain at increased risk of vascular calcification and subsequent cardiovascular death. Vitamin K-deficiency is common among KTR, which likely contributes to increased vascular calcification. We therefore studied the effects of vitamin K-supplementation on calcification propensity, vascular stiffness and vitamin K-status in KTR.
Methods: Between September 2020 and July 2021, we recruited clinically stable KTR for this single-center, parallel-group, randomized, double-blind, placebo-controlled trial. Vitamin K-deficiency was the main inclusion criterion, and was defined as a previously measured dephosphorylated uncarboxylated matrix gla protein (dp-ucMGP) > 500 pmol/L. Patients using a vitamin K-antagonist were excluded. Participants were randomized 1:1 to vitamin K2 (menaquinone-7, 360 µg once daily) or placebo for 12 weeks. The primary endpoint was serum calcification propensity (calciprotein particle maturation time, T50), and the main secondary endpoint was vascular stiffness (pulse wave velocity). Further secondary endpoints included dp-ucMGP and the ratio of uncarboxylated osteocalcin over carboxylated osteocalcin (ucOC/cOC) as markers of vitamin K-status, vitamin K-uptake and biological activity of vitamin K. Treatment effects were assessed by comparing changes in primary and secondary endpoints between treatment and placebo arms using T-tests or Mann-Whitney U-tests, depending on distribution.
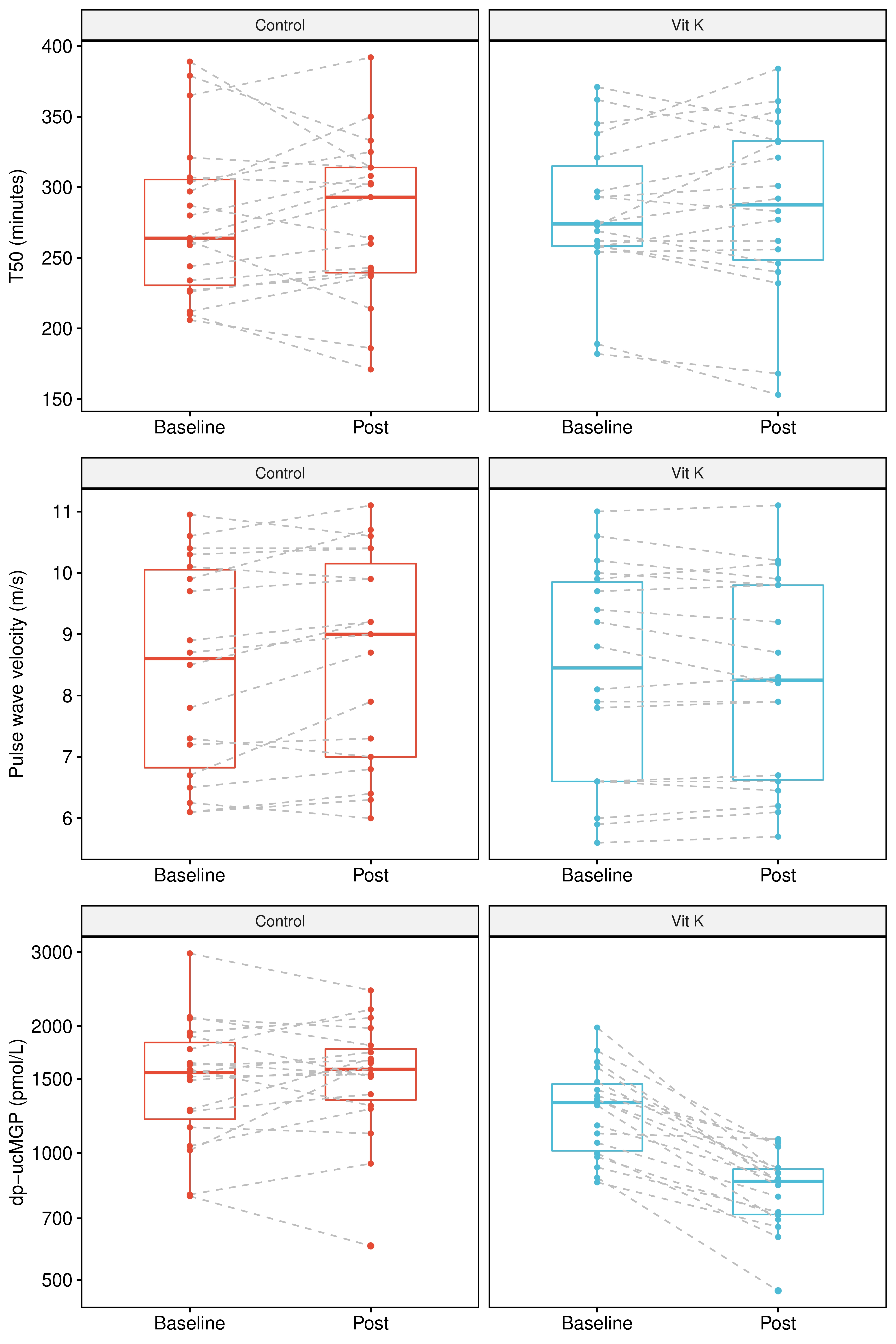
Results: Forty KTR (35% female, mean age 57 ± 13 years; median 9 [IQR: 5 to 15] years after transplantation) were randomized. The treatment groups (both N=20) were comparable at baseline. We observed no effect of vitamin K-supplementation on calcification propensity (change in T50, placebo: +0.8 ± 34.4 minutes; treatment: +2.3 ± 27.4 minutes; p=0.88), while we did observe a significant treatment effect on vascular stiffness (change in pulse wave velocity, placebo: +0.25 ± 0.43 m/s; treatment: -0.06 ± 0.26 m/s, p=0.010, Figure). Uptake and biological activity of vitamin K2 were confirmed by strong decreases in dp-ucMGP (relative change: -34% [IQR: -46% to -24%]) and ucOC/cOC ratio (relative change: -49% [IQR: -65% to -21%]) in patients in the treatment arm.
Discussion: Although vitamin K2-supplementation did not alter calcification propensity, we observed a significant beneficial treatment effect on vascular stiffness, suggesting that vitamin K2 may have local vascular effects in the absence of detectable systemic effects on calcification propensity. These results set the stage for studies addressing long-term intervention with vitamin K2 supplementation in KTR.
The study was supported by the Dutch Kidney Foundation (Nierstichting). The subsidizing party had no role in the design or conduction of the study, or in the writing and publication process.

right-click to download
