
Outcomes of sodium–glucose cotransporter 2 inhibitors and glucagon-like peptide-1 receptor agonists in diabetic kidney transplant recipients
Tarek Mahmoud1, Jude Yagan1, Amal Hasan2, Osama Gheith1, Mohamed Mostafa1, Suzzan Rida1, Nabil El-Serwi1, Mohamed Shaker1, Mahmoud Khalid1, Prasad Nair1, Torki Alotaibi1.
1Nephrology, Organ Transplant Center, Sabah, Kuwait; 2Clinical research, Dasman Diabetes Center, Kuwait, Kuwait
Introduction: The impact of the new glucose lowering therapies, sodium–glucose cotransporter 2 inhibitors (SGLT2i) and Glucagon-like peptide-1 receptor agonists (GLP-1RA), on managing patients with type II diabetes mellitus is very impressive with clear evidence of improving the cardiovascular and renal outcomes. Kidney transplant recipients (KTRs) with diabetes mellites have higher risks for cardiovascular and renal morbidities and mortalities. The use of these drugs was limited by the postulated fear of their side effects on renal graft outcomes. Few case series and small prospective trials in KTRs were published in the literature. We retrospectively assessed the safety and short-term outcomes of these drugs in our patients.
Patients and Methods: We collected data from records of 98 diabetic KTRs who received SGLT2I and another 41 who received GLP-1RA for at least 3 months. We compared them to a matched group of 70 diabetic KTRs on standard of care (SOC) therapy. Patients were at least 3 months post-transplant with stable renal function at the time of inclusion and estimated glomerular filtration rate (eGFR) of at least 25 ml/min/1.73m2. The groups were matching regarding age, gender, body mass index (BMI), type of donor, immunosuppression, post-transplant duration and type of diabetes. HbA1c was higher in both study groups compared to control (8.0% versus 7.2%). Follow up data for one year was recorded.
Results: HbA1c dropped by 0.4% in both SGLT2i (p=0.0001) and GLP-1RA (p=0.0003) groups compared to a reduction by only 0.05% in the control group. BMI decreased by 0.32 for the SGLT2i group (p=0.0450) and by 0.34 for the GLP-1RA group (p=0.0105) while increased by 0.015 for the control group. There was a tendency for better estimated glomerular filtration rate (eGFR) towards the end of the year in both study groups though it was not statistically significant except for the group of KTRs on SGLT2i with eGFR more than 90 ml/min (p=0.0135). A dip in eGFR was observed in KTRs on SGLT2i at one and 3 months. Albuminuria was significantly reduced at 12 months in SGLT2i group by a median of 28 mg/mmol of creatinine (p=0.0095) and by 20 mg/mmol creatinine in GLP-1RA group (p=0.0072) compared to increase by 3.4 mg/mmol creatinine in the control group. Side effects of the drugs were minimal and comparable to the control group.
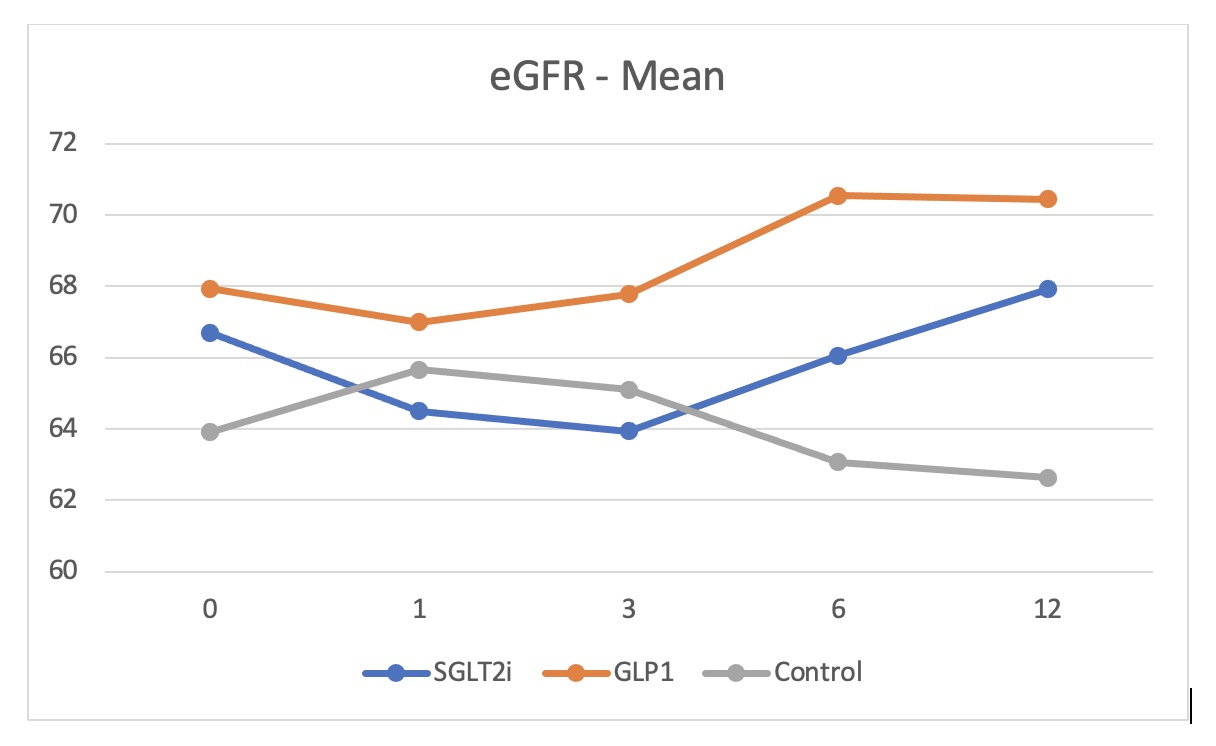
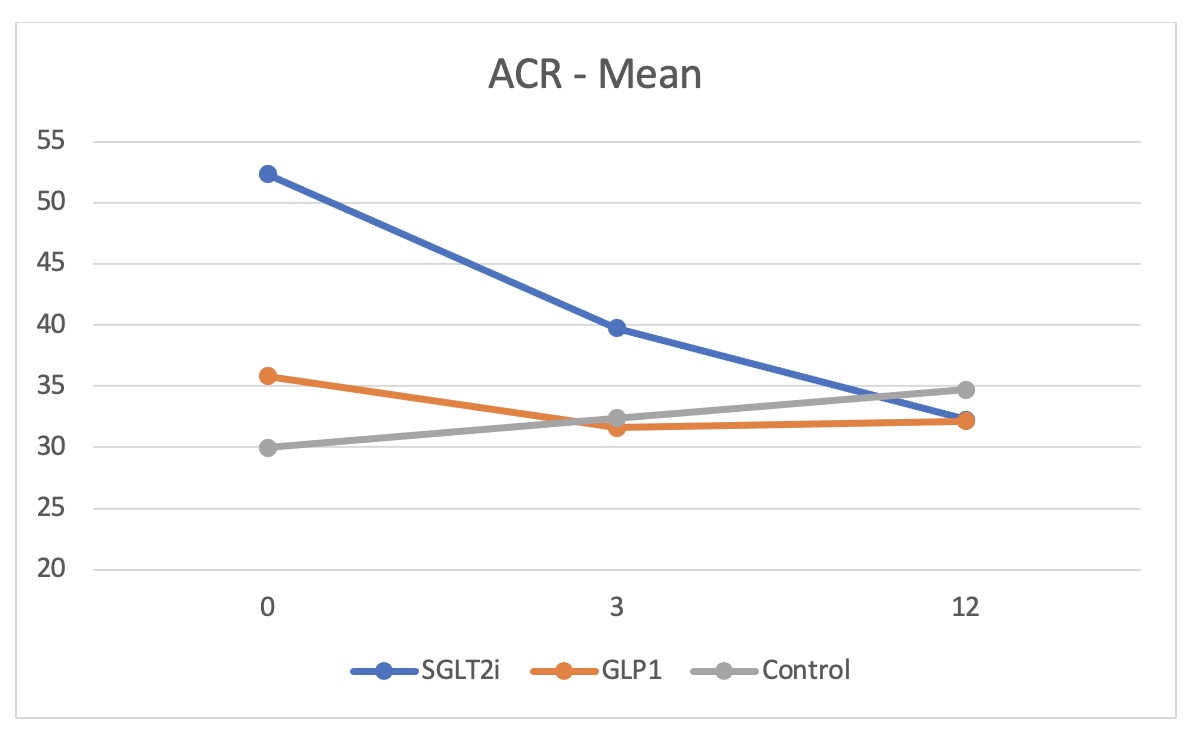
Conclusion: Use of SGLT2i and GLP-1RA is safe in diabetic kidney transplant recipients and is associated with better outcome with no increased side effects. Conducting randomized control trials is extremely required to confirm these findings and establish the guidelines.

right-click to download
