Association of skin cancer with the use of azathioprine and mycophenolate mofetil in kidney transplant recipients
Hatem Ali1, David Briggs2, Nithya Krishnan1.
1Renal department, University Hospitals of Coventry and Warwickshire, COVENTRY, United Kingdom; 2NHSBT, Birmingham, United Kingdom
Introduction: Skin cancer, especially squamous cell carcinoma (SCC), is the most common type of cancer among kidney transplant recipients. SCC is more likely to spread (metastatic SCC), which can result in the patient's death. The cause of skin cancer among transplant patients is thought to be due to immunosuppressive therapy. These drugs must be used for a long time to prevent organ rejection, but they limit the immune system's ability to destroy UV-damaged cells, allowing these damaged cells to grow into malignancies. In transplant patients, ultraviolet (UV) exposure is a crucial contributor to the development of skin cancer. Our study aims to assess the Association of Skin Cancer with the use of Azathioprine and Mycophenolate mofetil in Kidney Transplant Recipients.
Methodology: Data of 941 patients who had renal transplantation between 1998 and 2018 in University Hospitals of Coventry and Warwickshire were retrospectively reviewed. Data contained information about age, gender ethnicity, type of transplant, the previous number of transplantation, cancer status, medication, dose, duration of medication, skin cancer diagnosis results, non-skin cancer diagnosis results, treatment start date, treatment follow-up with a time gap of 6months, 1, 5, 10, 15 and 20 years, and type of treatment they had. Exclusion criteria: HLA incompatible transplant, patients with pre-transplant skin cancer, patients with incomplete data, and patients who switched from azathioprine to Mycophenolate mofetil or vice versa. Cox proportional hazard regression analysis was used to evaluate factors associated with a higher risk of skin cancer. Analysis of variance (ANOVA) test was used to compare the relationship between Azathioprine versus Mycophenolate Mofetil and subtypes of skin cancer.
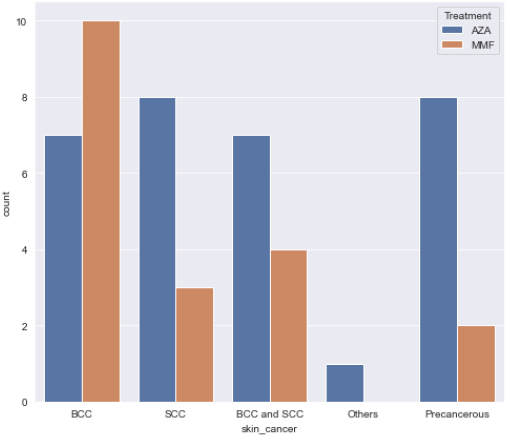
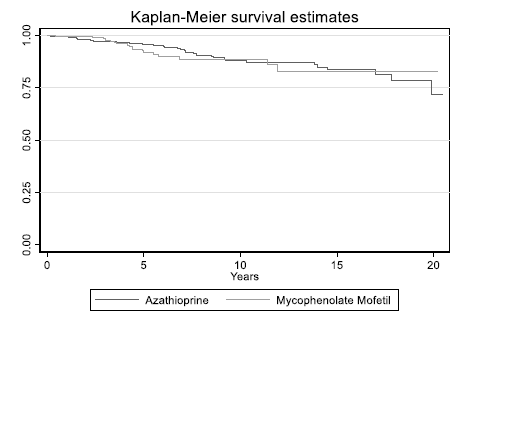
Results: Overall, there was no significant difference between Azathioprine and Mycophenolate Mofetil in terms of occurrence of skin cancer (HR=1.05, P=0.87, 95%CI: 0.56-1.94). An increase in the number of previous transplants was associated with a higher risk of skin cancer (HR=2.48, P<0.01, 95%CI: 1.23-5.01). Patients with age>50 years old were associated with a higher risk of skin cancer (HR=4.98, P<0.01). On further subgroup analysis using ANOVA , Mycophenolate Mofetil was associated with a higher risk of developing basal cell carcinoma (BCC) (52.6%) in comparison to Azathioprine (22.6%) with a P value<0.05. Azathioprine was associated with a higher risk of developing squamous cell carcinoma (25.8%) in comparison to Mycophenolate Mofetil (15.8%) with P value<0.05.
Conclusion: There was no significant difference between Mycophenolate Mofetil and Azathioprine in terms of occurrence of skin cancer .Increase in age, and the number of the previous transplant are associated with a higher risk of skin cancer. Azathioprine is associated with a higher risk of developing SCC while Mycophenolate Mofetil is associated with a higher risk of developing BCC.

right-click to download
