Tolerant kidney transplant recipients display a unique CTLA-4 dominant urinary cell mRNA signature
John Lee1, Joseph Leventhal2, Carol Li1, Clayton Hughes1, Ruchuang Ding1, Suzanne Ildstad3, Manikkam Suthanthiran1.
1Medicine / Nephrology and Hypertension, Weill Cornell, New York, NY, United States; 2Surgery / Organ Transplantation, Northwestern Medicine, Chicago, IL, United States; 3Talaris Therapeutics, Louisville, KY, United States
Introduction: Urinary cell mRNA profiling provides an unparalleled window into the intra-graft events in kidney transplant recipients. Utilizing this powerful molecular tool, we aimed to characterize mRNA signatures associated with human kidney allograft tolerance induced with tolerogenic CD8+/TCR- facilitating cells (FCR001) and a nonmyeloablative conditioning regimen.
Methods: We developed a custom panel of mRNAs encoding immunoregulatory proteins implicated in allograft tolerance and rejection and measured absolute levels of urinary cell mRNAs using preamplification enhanced real time quantitative PCR assays. We quantified urinary cell mRNA levels in 28 biopsy-matched urines from 14 FCR001-tolerant patients with durable chimerism and off immunosuppression (FCR Durable Cohort); 43 biopsy-matched urines from 34 kidney allograft recipients prospectively enrolled in the Clinical Trials in Organ Transplantation-04 (CTOT-04) and with biopsies with acute cellular rejection (TCMR Cohort); and 161 biopsy-matched urines from 124 kidney transplant recipients prospectively enrolled in CTOT-04 and with biopsies without rejection features (No Rejection Cohort).
Results: Urinary cell mRNA profile of the FCR Durable Cohort was unique and discriminated the tolerant patients from the patients with TCMR biopsies and the patients with No Rejection biopsies. Absolute levels of urinary cell mRNAs, summarized in Table 1, show that the median level of CTLA-4 mRNA is significantly higher in the FCR Durable Cohort (median 439 copies per microgram of RNA) than in the TCMR cohort (135 copies) or the No Rejection Cohort (13 copies) (Kruskal Wallis [KW] P= 8x10-21). In contrast, urinary cell level of mRNA encoding cytotoxic attack molecule granzyme B was significantly lower in the FCR Durable Cohort (median 106 copies) than in the TCMR cohort (3304 copies) or the No Rejection Cohort (337 copies) (KW P= 1x10-11). The differential gene expression pattern resulted in the ratio of CTLA-4 mRNA to granzyme B mRNA to be significantly higher in the FCR Durable Cohort (5.62) than in the TCMR Cohort (0.03) or the No Rejection Cohort (0.04) (KW P= 1x10-14). Urinary cell levels of mRNA for CD3E, perforin, IP-10, FoxP3, and TGFB1 in the FCR Durable Cohort were significantly lower in the TCMR Cohort and mostly like the profile in the No Rejection Cohort. Comparison to an FCR Transient Cohort (11 biopsy-matched urines from 3 patients conditioned with the FCR001 regimen but with transient chimerism and on immunosuppression) revealed a higher ratio of CTLA-4 mRNA to granzyme B mRNA in the FCR Durable Cohort compared to the FCR Transient Cohort (5.62 vs. 1.99,P=0.21).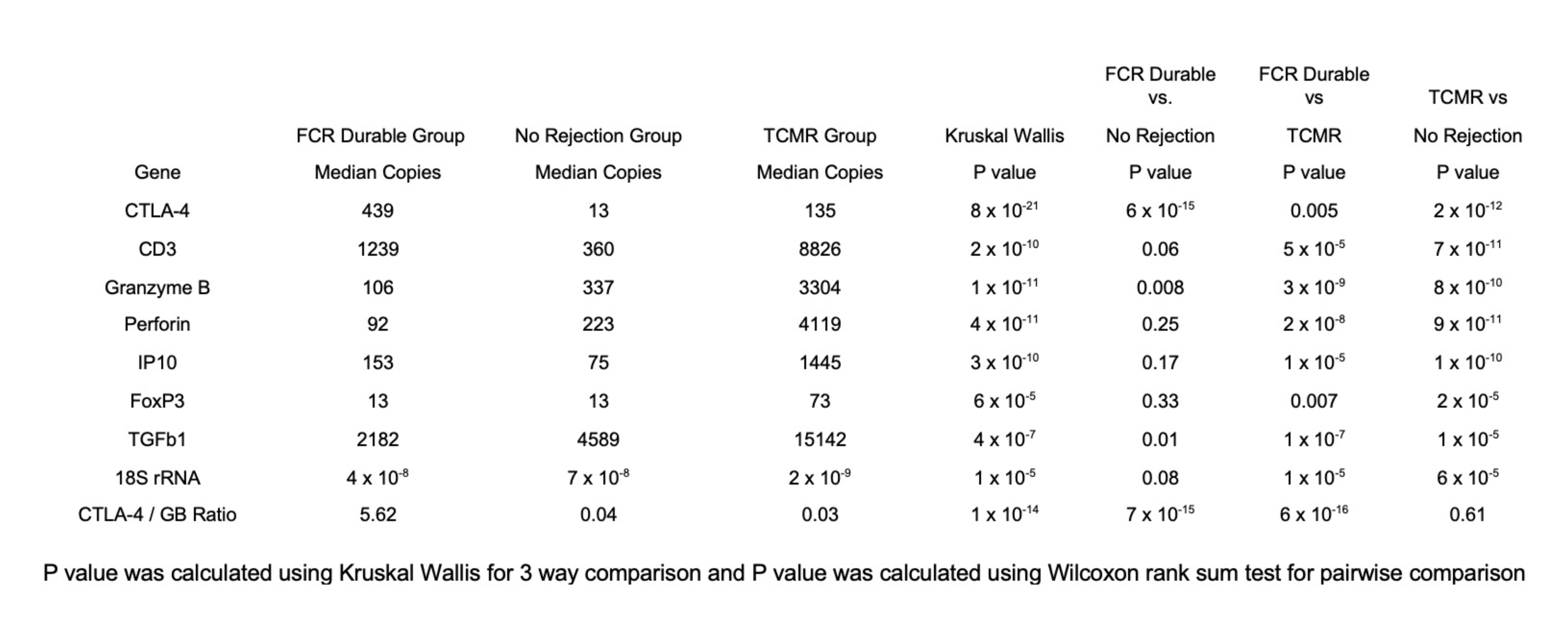
Conclusion: Kidney allograft tolerance induced with CD8+/TCR- facilitating cells and a nonmyeloablative conditioning regimen is associated with a tolerogenic signature of enhanced expression of CTLA-4 mRNA and decreased expression of mRNA for granzyme B and perforin.
The research was supported, in part, by NIH grants U01AI63589 and R37AI051652.

right-click to download
