Dyslipidemia in kidney transplant recipients: Is it time for a change in strategy?
Jay Pandav1, Rohan Saranu1, Saif Muhsin1, Kassem Safa1, Amtul Aala2, Steven Gabardi1, Anil Chandraker1.
1Nephrology, Brigham and Women's Hospital, Boston, MA, United States; 2Nephrology, Beth Israel Deaconess Medical Center, Boston, MA, United States; 3Nephrology, Massachusetts General Hospital , Boston, MA, United States
Introduction: Dyslipidemia is common in kidney transplant recipients (KTRs), and is associated with a more than 20-fold higher risk of major adverse cardiovascular events compared to the general population. Although KDIGO 2013 guidelines suggest that all KTRs be treated with statins, they are poorly tolerated due to myopathy, hepatotoxicity, and interaction with immunosuppression medications leading to poor compliance. The introduction of the novel lipid-lowering Proprotein convertase subtilisin/Kexin type 9 (PCSK-9) inhibitors presents an opportunity to treat a significant unmet need in KTRs.
Methods: Here we report findings of 3 studies related to hyperlipidemia in KTRs. We conducted a single-center cross-sectional analysis of 250 KTRs about the prevalence of hyperlipidemia, ASCVD scores, frequency of lipid monitoring, and statin use. After our analysis, we performed a nationwide survey using a questionnaire on a lipid management strategy that was sent out to KTR providers and pharmacists. We also conducted a prospective study evaluating PCSK-9 inhibitor (Evolocumab) in the treatment of LDL-cholesterol (LDL-C) levels for KTRs. Patients included in the study were started on Evolocumab 140 mg subcutaneously every 2 weeks and their lipids were checked at 3 months.
Results: Our institutional study showed suboptimal lipid control in KTRs with only about 52.3% getting optimal monitoring (Figure 2). This was also seen in the national survey involving 34 centers, where we found disparities and a lack of general consensus in lipid management (Figure 3). With regards to PCSK-9 inhibitor study, all 17 patients with 3 monthly follow-up (n=44 enrolled to date) showed a significant drop in their LDL-C levels (35% to 90%). There was also a remarkable reduction in ASCVD scores. The mean eGFR for all patients was stable (Figure 4). Overall, Evolocumab was well tolerated by all patients.
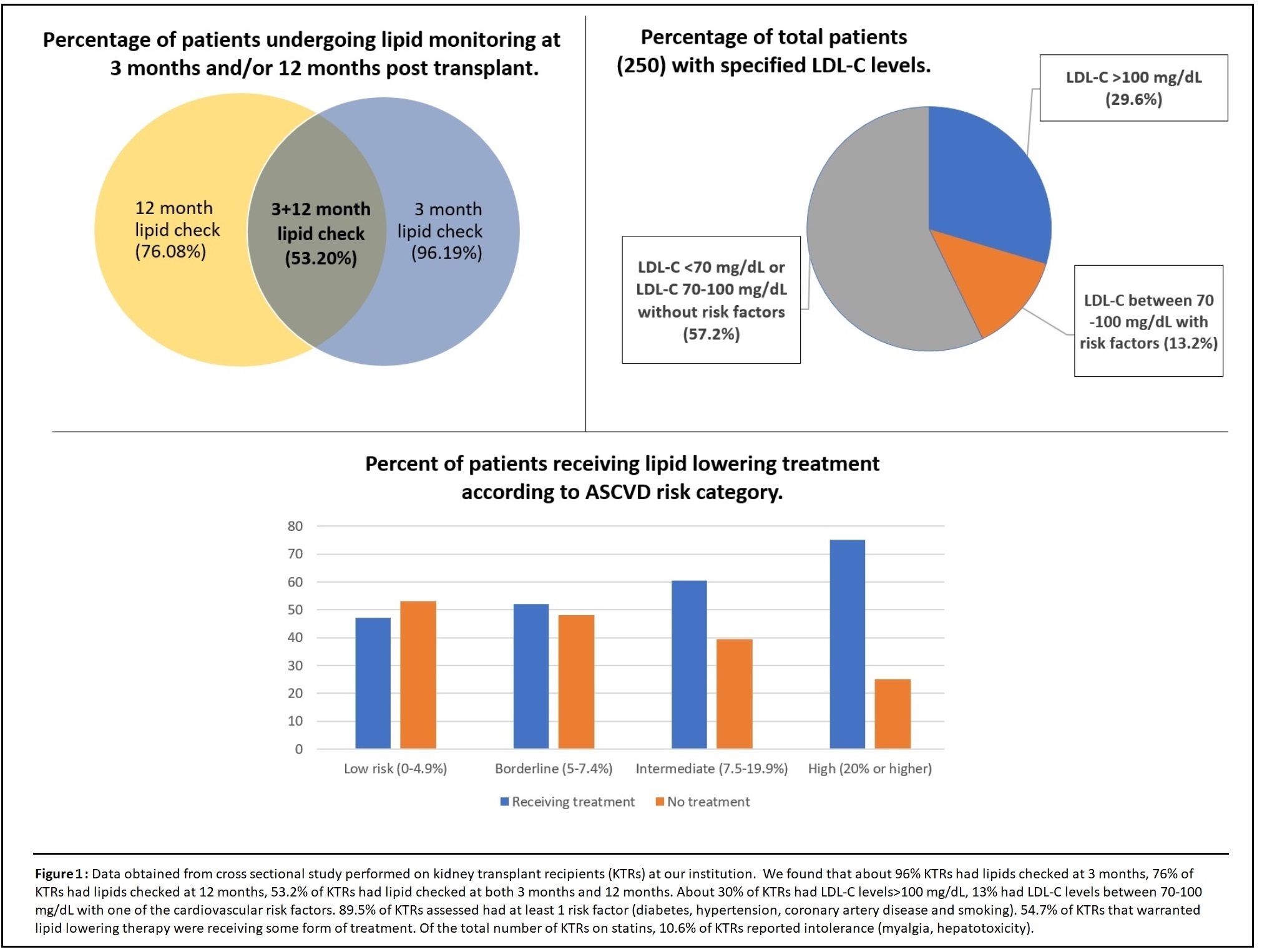
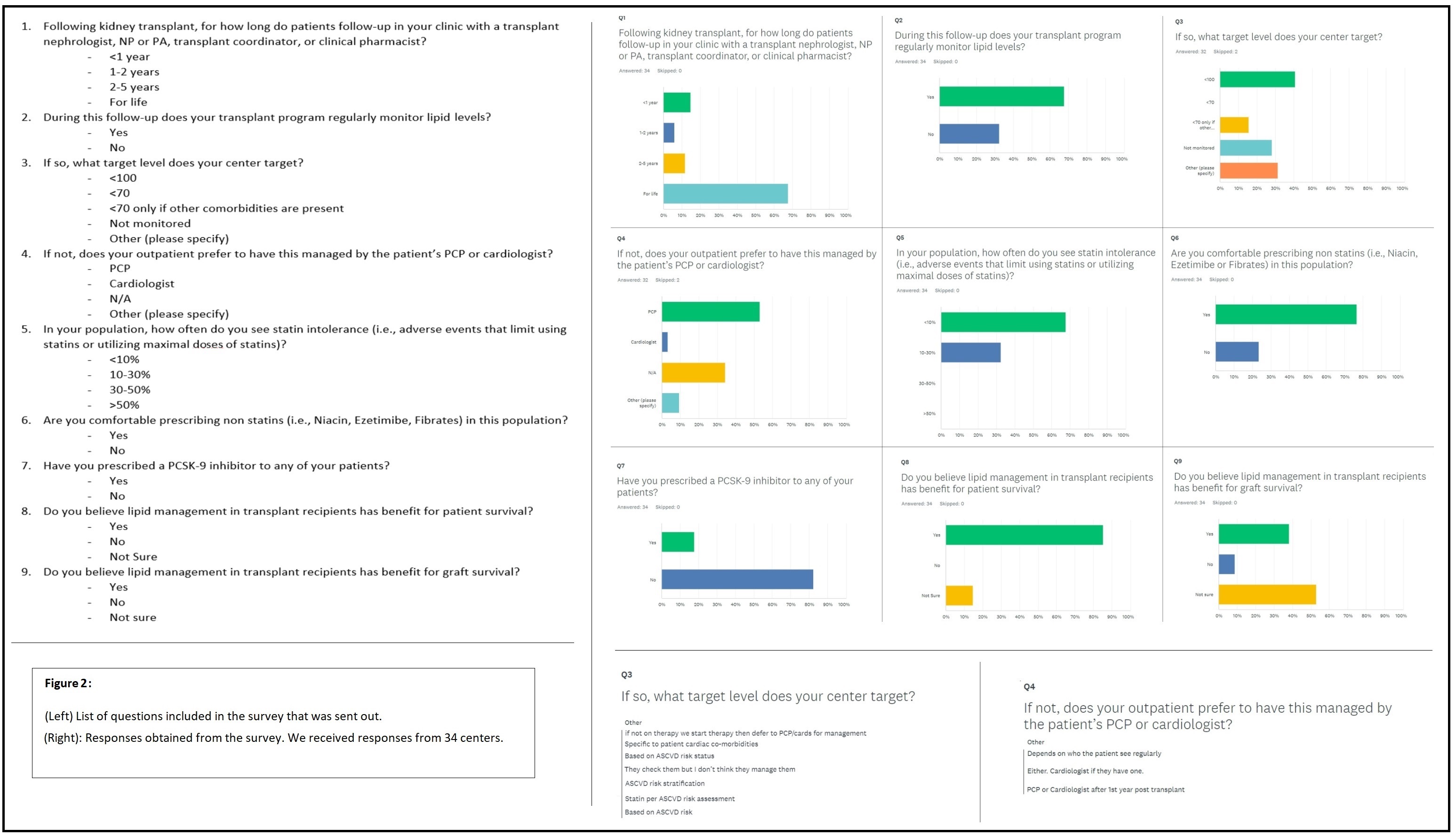
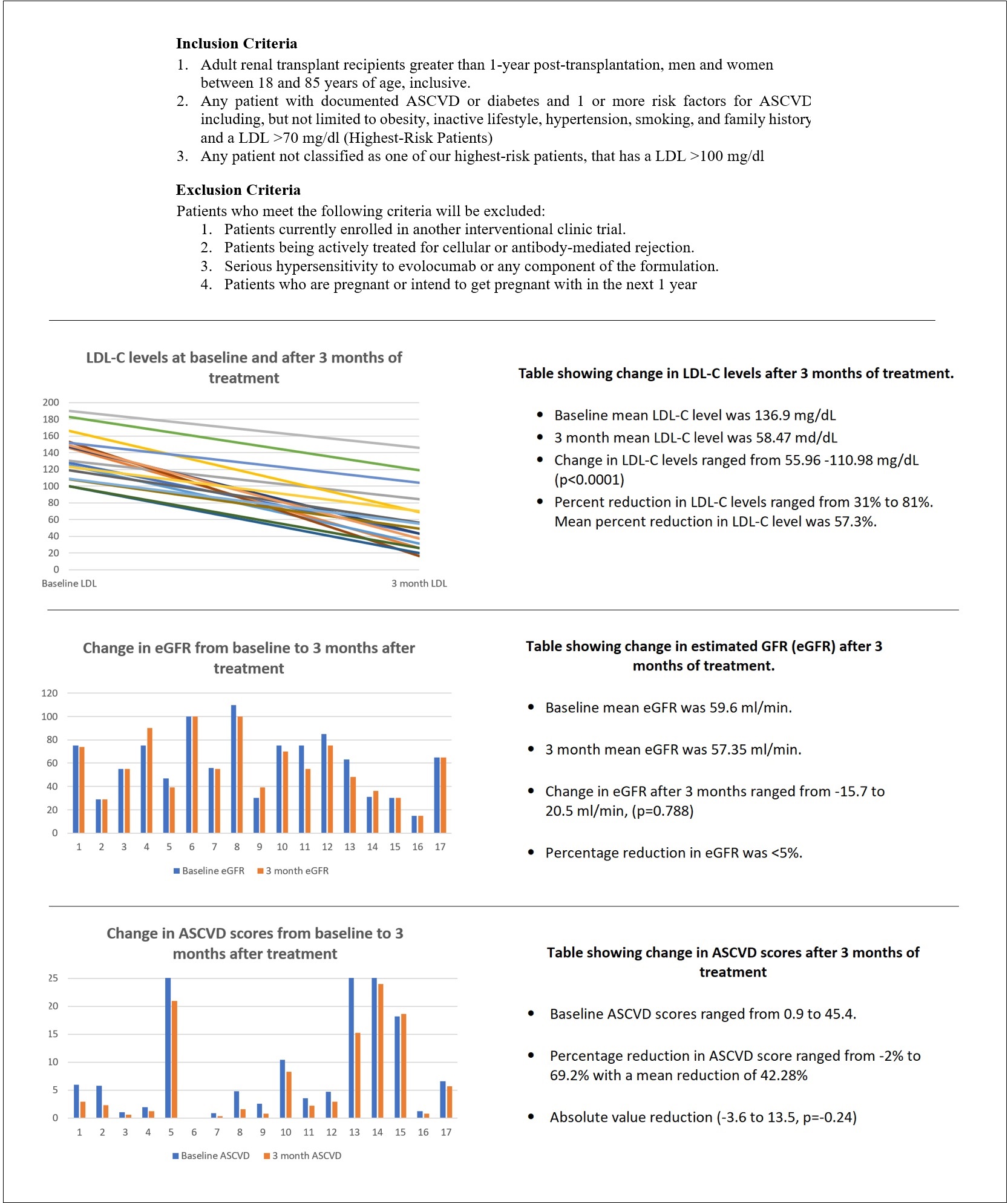
Discussion: Sub-optimal lipid control found at our institution, along with the disparities in the survey responses indicate that there is no uniform strategy in lipid management in KTRs. This could be due to a lack of strong evidence on which the 2013 KDIGO guidelines are based. There is a need for long-term studies to assess the benefits of lipid management on patient and graft survival in KTRs and the development of a clearer, more standardized approach to the management of lipids. In terms of treatment, PCSK-9 inhibitors seem to be very effective for the reduction of LDL-C levels in KTRs. They have a favorable side effect profile and do not interact with immunosuppressive medications. Unlike cardiovascular benefits shown by statins in long-term studies, there is no data regarding PCSK-9 inhibitors as of yet. Given the challenges faced with statins, PCSK-9 inhibitors could be considered the therapy of choice for statin-intolerant patients, if supported by evidence of beneficial cardiovascular outcomes.
"Study of safety and efficacy of PCSK-9 inhibitor (Evolocumab) in kidney transplant recipients." is funded by AMGEN.

right-click to download
