Prebiotic supplementation in kidney transplant recipients for preventing infections and gastrointestinal upset: a randomized controlled feasibility study
Samuel Chan1,2,3, Carmel M. Hawley1,2,3, Elaine M. Pascoe1,2,3, Christopher Z. Cao1, Scott B. Campbell1,2,3, Katrina L. Campbell4,5, Ross S. Francis1,2,3, Rachael Hale1, Nicole M. Isbel1,2,3, Mark Morrison6, David W. Johnson1,2,3.
1Nephrology, Princess Alexandra Hospital, Brisbane, Australia; 2Australasian Kidney Trials Network, The University of Queensland, Brisbane, Australia; 3Translational Research Institute, Brisbane, Australia; 4Centre for Applied Health Economics, Menzies Research Institute, Griffith University, Brisbane, Australia; 5Healthcare Excellence and Innovation, Metro North Health, Brisbane, Australia; 6Diamantina Institute, Faculty of Medicine, The University of Queensland, Brisbane, Australia
Background: Modulating the large intestinal microbiome of kidney transplant recipients (KTR) may reduce infectious complications. The aim of this study was to assess the feasibility of a randomized controlled trial of prebiotics in reducing infections and gastrointestinal symptoms in KTR.
Methods: Acute KTR were recruited to a double-blind, placebo-controlled, randomized trial at the Princess Alexandra Hospital. Patients were provided with prebiotics or placebo for 7 weeks. The primary outcome was feasibility, defined as recruitment of ≥80% of eligible people within 6 months. Secondary outcomes included adherence and tolerability, participant retention in trial, proportions of participants providing serum and stool specimens, self-reported quality of life (QOL), gastrointestinal symptoms and infection events.

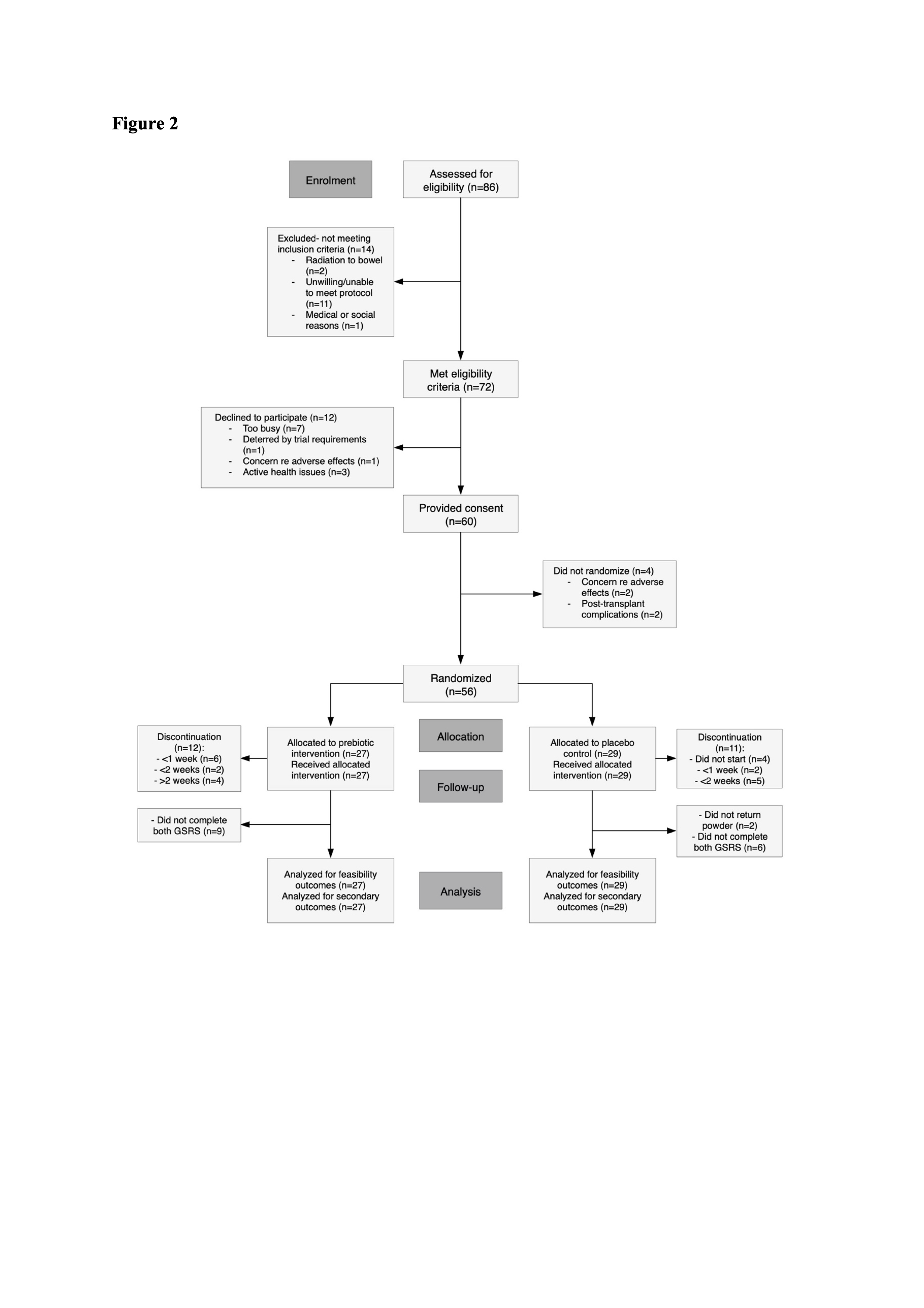
Results: During the 7-week period, 72 patients met eligibility criteria, of whom 60 (83%) consented to participate (mean±SD age 53±12 years; 62% males). Fifty six (78%) participants were randomized (27 intervention and 29 control). While participants receiving intervention experienced reduced gastrointestinal symptoms (-0.28 [IQR -0.67 to 0.08] vs -0.07 [IQR -0.27 to 0], p=0.03), both control and intervention groups were similar in adherence (67% vs. 72%, p=0.36), tolerability (56% vs. 62%, p=0.64), QOL (-0.2 [IQR -0.6 to 0] vs. –0.2 [IQR -0.8 to 0], p=0.82) and infection events (33% vs. 34%, p=0.83). Blood and stool samples were collected from ≥90% of participants in both groups.
Conclusions: It is feasible to recruit and retain acute KTR in a randomized placebo-controlled trial examining the effect of prebiotics on infections and gastrointestinal symptoms. This study also showed that prebiotics significantly reduced gastrointestinal symptoms.
Metro South Research Support Scheme Project Grant .

right-click to download
