Immunogenicity of the inactivated SARS-CoV-2 vaccine (BBIBP-CorV; Sinopharm) and short term clinical outcomes in vaccinated solid organ transplant recipients: a prospective cohort study
Mojtaba Shafiekhani1, Hamed Nikoupour1, Mahtabsadat Mirjalili1, Jamshid Roozbeh1, Siavash Gholami1.
1Shiraz Transplant Center,Abu-Ali Sina Hospital, Shiraz university of medical sciences , Shiraz, Iran (Islamic Republic of)
Introduction: Immunocompromised patients have lower seroconversion rate to Coronavirus disease 2019 (COVID-19) vaccination due to immune system dysfunction. So far, the majority of studies in this area are conducted on messenger ribonucleic acid (mRNA) based vaccines. The aim of this study is evaluation of humoral immune response and in transplant patients and short term clinical outcomes in transplant patients vaccinated with SARS-CoV-2 Vaccine (BBIBP-CorV).
Methods: All patients older than 18 years old who had been transplanted more than six months prior to recruitment were included in this prospective cohort conducted in Shiraz transplant center as the largest transplant center in Asia from March to December, 2021. The patients received two doses of Sinopopharm 4 weeks apart. The immunogenicity was evaluated through assessment of antibodies against the receptor-binding domain (RBD) of SARS-CoV-2 by enzyme-linked immunosorbent assay (ELISA) method 4 weeks after the first and second dose of vaccine. The patients were followed up weekly up to six months after the second dose monthly up to six months after second dose and were evaluated for occurrence of vaccine adverse events, or getting COVID-19, hospitalization and laboratory parameters. The clinical and laboratoty data of patients was recorded and analyzed by statistical tests.
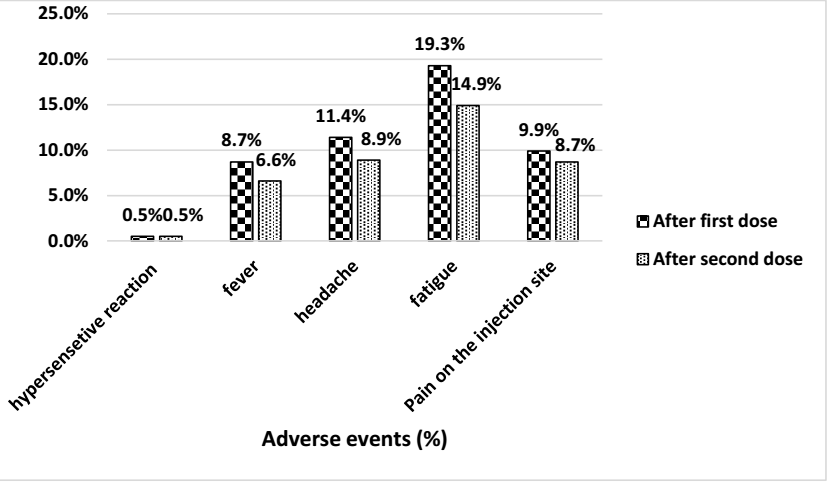
Results: Totally, 921 transplant patients (665 kidney transplantation, 221 liver transplantation, 28 simultaneous pancreas kidney (SPK) and 7 heart transplant recipients). 115 (12.5%) and 239 (26%) patients had acceptable anti S-RBD IgG levels 4 weeks after the first and second dose, respectively. After omitting COVID19 cases with positive PCR test within 6 months before vaccination, 104 (12.6%) and 211 (25.5%) patients had acceptable anti S-RBD level 4 weeks after the first and second dose. Totally, 80 patients (8.68%) got infected with COVID-19 after vaccination.45 (4.9%) patients were admitted in the hospital due to COVID-19 after receiving the second dose of vaccine. None of the patients died during 6 months follow up period. 24 (10.9%) ones developed liver enzyme elevation Also, rise in serum creatinine was observed in 86 (13.5%) kidney transplant patients. Two of SOT recipients experienced biopsy proven rejection which managed by high dose of corticosteroid without any graft loss episodes.
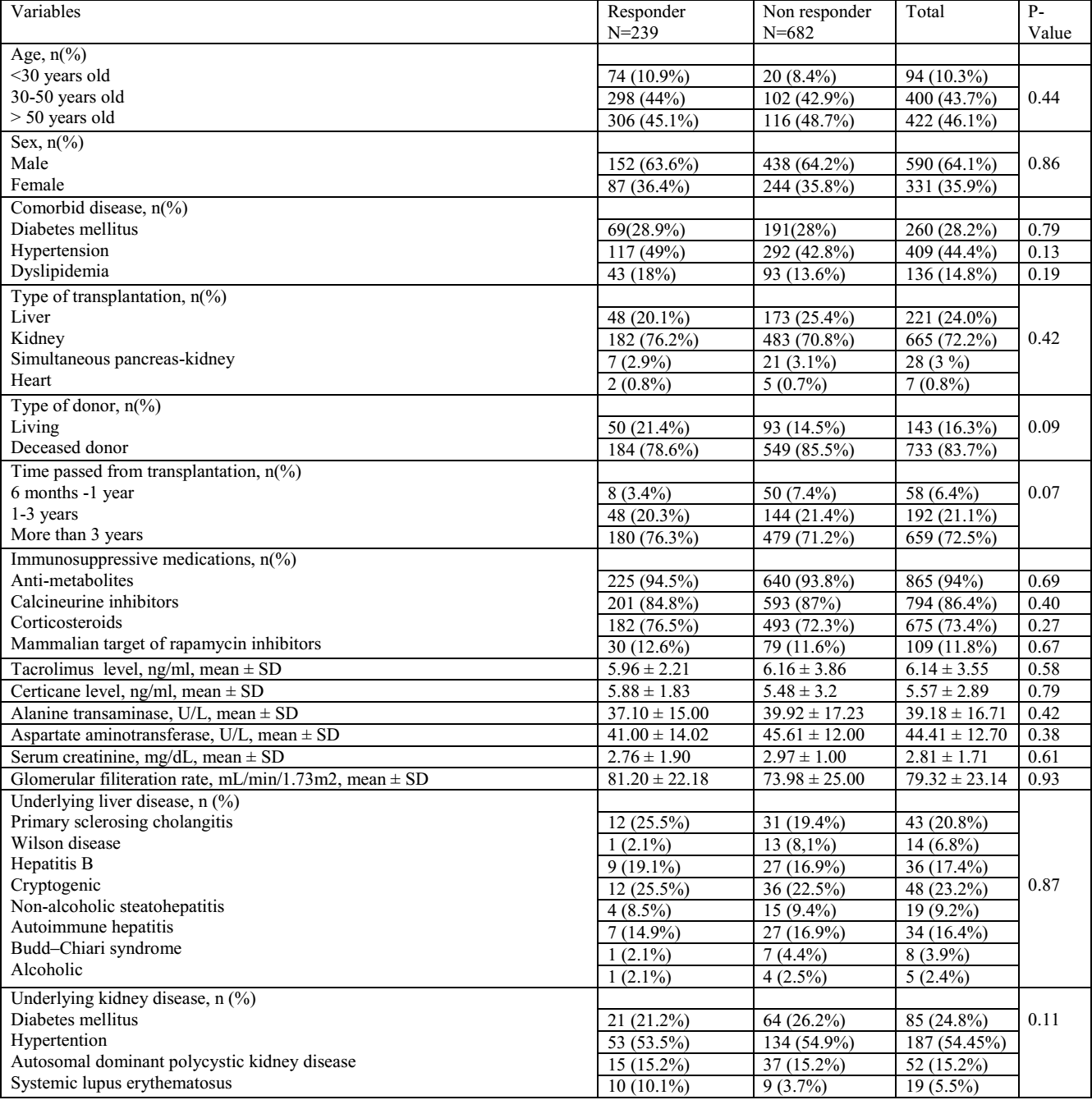
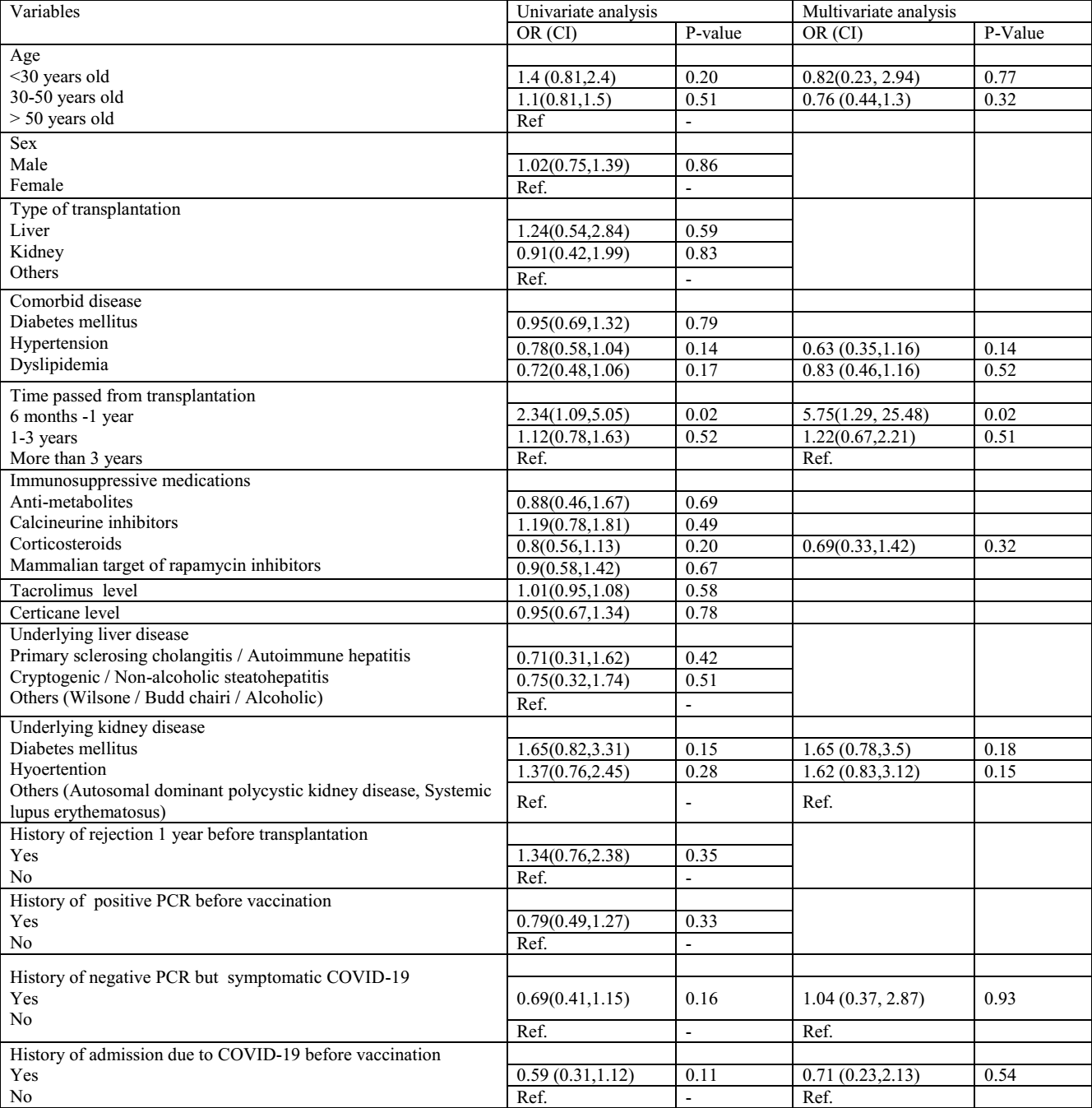
Conclusion: The results of our study showed that humoral response rate to Sinopharm vaccine was low in transplant patients. Short time from transplant was only effective factor on low seroconversion rate in transplant patients due to the high dose of immunosuppressive medications in this time frame. It is recommended that the third dose of vaccine, particularly from a different vaccine platform from the inactivated vaccine be administered in transplant patients.

right-click to download
