Physicians’ opinions regarding low-level Epstein-Barr virus DNA levels from PCR testing
Akos Somoskovi1, Poulami Chakraborty1, Daniel Jarem1, Paul Baum1.
1Roche Molecular Systems, Inc., Pleasanton, CA, United States
Introduction: Epstein Barr virus (EBV) DNA measurement supports diagnosis and management of EBV-associated complications in transplant recipients. Laboratory-developed EBV DNA tests can be variable between laboratories and relatively insensitive. The availability of more sensitive, consistent and accurate tests, with results that are traceable to the WHO International Standard and are reported IU/mL of plasma, raises questions regarding the clinical importance of results in lower titer ranges that are newly detectable. Commercially available EBV DNA tests now exhibit sensitivity and even linearity near or below 100 IU/mL in plasma. With the advancement of more sensitive diagnostics, the clinical significance of low-level EBV DNA test results should be considered, however, no clear clinical consensus is currently available to help interpret these results, and it is unclear what actions clinicians may take when presented with them. We undertook a survey of practicing adult and pediatric transplantation clinicians in order to characterize their likely therapeutic and diagnostic decisions in response to theoretical scenarios involving low-level EBV DNA test results.
Methods: Fifty-one clinicians who manage transplant recipients in the US, UK and Australia responded to a questionnaire presenting two hypothetical clinical scenarios involving low-level EBV DNA test results in adult and pediatric transplant patients. Scenario 1 included a detectable DNA level that was below the limit of quantitation. Scenario 2 involved low-level DNA results (150 vs. 400 IU/mL of plasma) from two different laboratories.
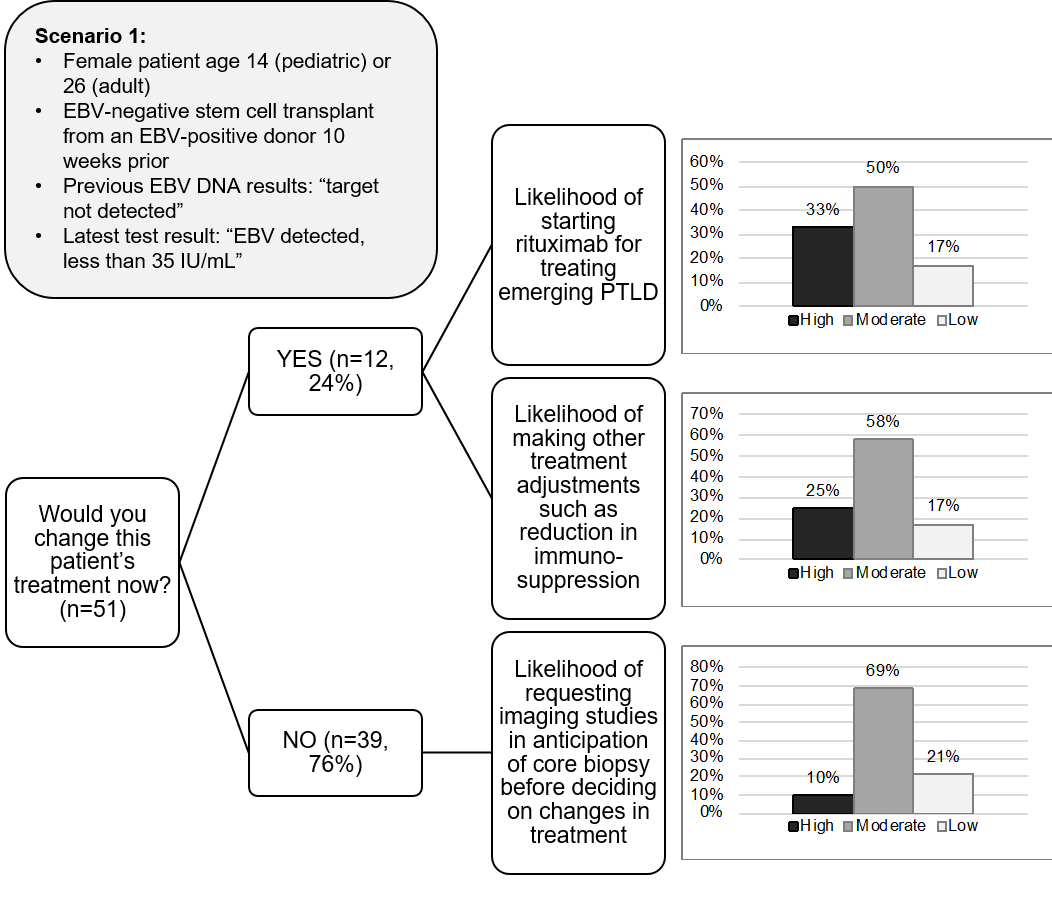
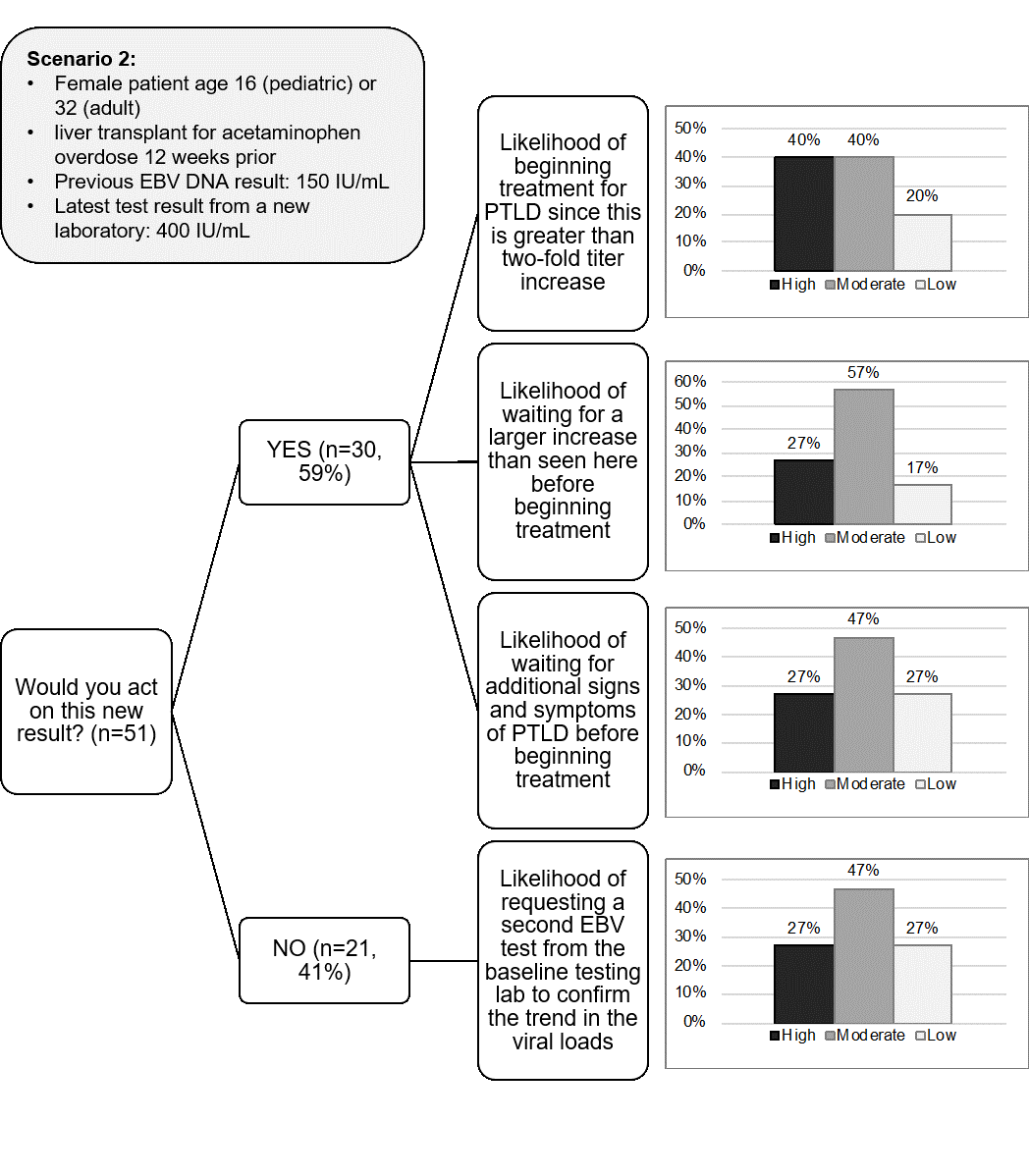
Results: Almost a quarter (24%) of clinicians would change treatment based on a single detectable EBV DNA result as presented in scenario 1 (Figure 1). Before changing treatment or ordering imaging studies in anticipation of possible core biopsy, the overwhelming majority would repeat EBV testing, mostly on a weekly basis. Most clinicians (59%) were likely to act based on the apparent increase in EBV DNA in scenario 2, with most intending to confirm the results through repeat testing to confirm the trend in viral load (Figure 2).
Conclusions: Adequate use and uptake of diagnostic testing in clinical medicine is often suboptimal leading to misinterpretation by clinicians and unnecessary diagnostic errors. Our findings reveal the impact that low-level EBV DNA plasma results may have on potential patient management approaches. In addition, the survey also showed marked differences in how clinicians would interpret and act on these results. This highlights that, in our theoretical clinical scenarios, there is no clear consensus regarding these potential and diverse interventions based on the different diagnostic and therapeutic approaches triggered by low EBV DNA plasma levels. These observed differences underline the need for more interpretive guidance to support the wider availability of more accurate, sensitive and standardized EBV DNA level monitoring assays.

right-click to download
