Healthcare resource utilization in transplant recipients with cytomegalovirus infection refractory to prior treatment with/without resistance receiving maribavir versus investigator-assigned therapy: exploratory analysis of the phase 3 SOLSTICE trial
Ishan Hirji1, Kim Cocks2, Alejandro Moreno-Koehler3, Aimee Sundberg1.
1Takeda Development Center Americas, Inc., Lexington, MA, United States; 2Adelphi Values, Bollington, United Kingdom; 3Adelphi Values, Boston, MA, United States
Introduction: Increased healthcare resource utilization (HCRU) has been associated with cytomegalovirus (CMV) infection following transplantation, in particular in patients (pts) requiring >1 antiviral course. In the Phase 3 SOLSTICE study (NCT02931539), maribavir (MBV) was superior to investigator-assigned therapy (IAT; val/ganciclovir, foscarnet, cidofovir) for CMV clearance at Wk 8 and clearance plus symptom control Wk 8 maintained through Wk 16 in transplant recipients with refractory CMV infection with/without resistance (R/R). This exploratory analysis of SOLSTICE evaluated HCRU for the MBV and IAT arms.
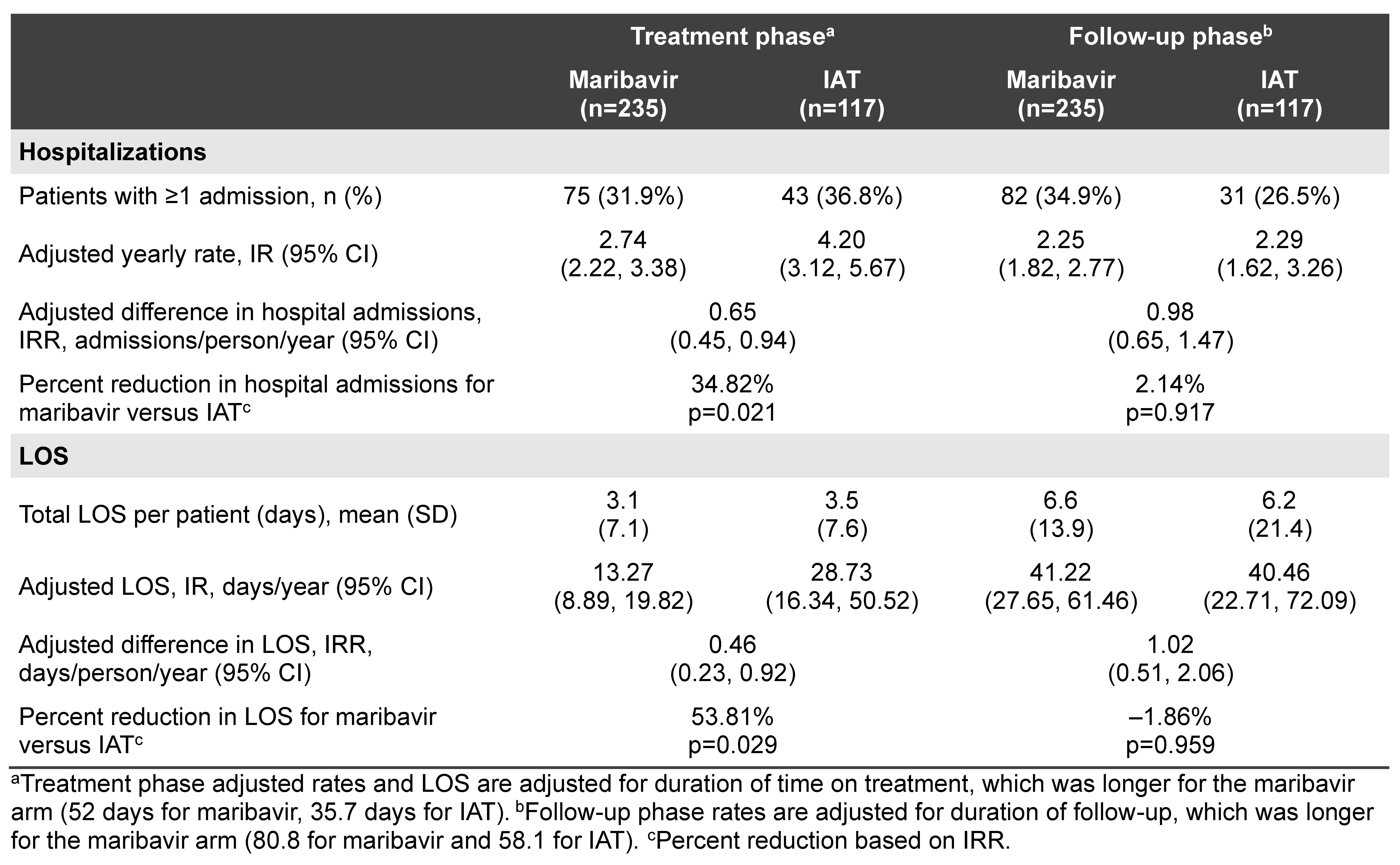
Methods: Transplant recipients with CMV R/R to prior treatment (failure to achieve >1log10 decrease in CMV DNA after ≥14 days, with/without genotyped resistance) were randomized 2:1 to MBV 400 mg BID or IAT for 8 wks with 12 wks of follow-up. After ≥3 wks of treatment, pts in the IAT arm with pre-specified criteria could enter a MBV rescue arm (8 wks’ MBV treatment, 12 wks’ follow-up). Data on hospital admissions were collected at each study visit and analyzed by treatment during the treatment and follow-up phases. Analyses included the number of pts with ≥1 hospitalization and length of hospital stay (LOS). Hospitalization rates and LOS (per person/year) were estimated using negative binomial models adjusting for exposure time. Adjusted incidence rates (IR), 95% CIs, IR ratios (IRR) and percent reduction in IRRs were calculated. Supplementary analyses described hospitalizations and LOS for the rescue arm and individual IAT groups.
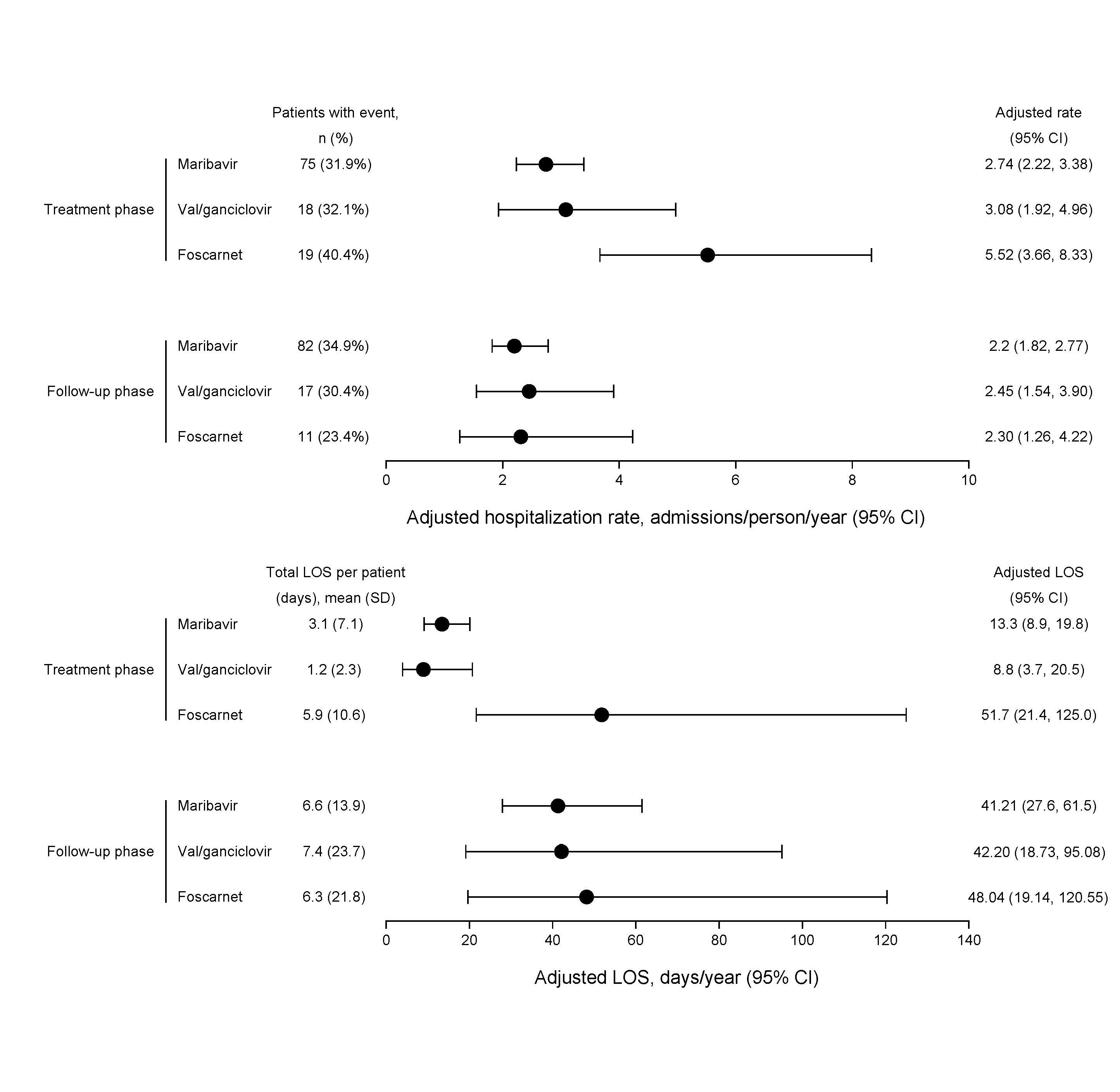
Results: In total, 352 pts were randomized (MBV: 235; IAT: 117), of whom 22 (18.8%) entered the MBV rescue arm. While on treatment, pts on MBV versus IAT had reductions of 34.8% in hospitalizations (p=0.021) and 53.8% in LOS (p=0.029; Table). Hospitalization rates were lower during the follow-up than treatment phase, with no differences between treatments in the follow-up (off-treatment) period (Table). The hospitalization rate in the IAT group pre-rescue with MBV (IR=6.98; 95% CI: 4.06, 12.03) was 2.54 times higher (IRR=2.54; 95% CI: 1.28, 5.04; p=0.008) than on or after rescue with MBV (IR=2.75; 95% CI: 1.81, 4.18); increased LOS pre-rescue was also noted (IRR=2.25; 95% CI: 0.72, 7.01) but not statistically significant. In the IAT arm, foscarnet pts tended to have higher hospitalizations (5.5 admissions/person/year) and longer LOS (51.7 days/person/year) during the treatment phase compared with patients on val/ganciclovir or maribavir (Figure).
Conclusion: Results from this analysis quantify the HCRU experience of pts requiring treatment for post-transplant CMV. Hospitalizations and LOS were lower for pts on MBV than IAT. Pts who received MBV rescue reported lower hospitalization rates on or after rescue than pre-rescue. Reducing hospitalizations is critical for alleviating disease burden to healthcare systems.
TCT2022 encore; DOI pending
This study was funded by Takeda Development Center Americas, Inc. Writing and editing support was provided by Caudex (UK), funded by Takeda Development Center Americas, Inc.

right-click to download
