Similar graft-versus-host disease (GVHD) incidence among unrelated and identical sibling donor transplantation with the use of anti-thymocyte globulin (ATG)
Agustina Cia1, Martin m Castro2, Alejandro Requejo1, Nicolas Fernandez Escobar1, Gonzalo Bentolilla3, Gregorio Jaimovich1,2.
1Bone Marrow Tranplantation Programme, Favaloro University Hospital, Caba, Argentina; 2Bone Marrow Tranplantation Programme, Sanatorio Anchorena, Caba, Argentina; 3Bone Marrow Tranplantation Programme, Fundaleu, Caba, Argentina
Introduction: GVHD is the main cause of morbidity and mortality associated with allogeneic hematopoietic stem cell transplantation (HSCT) with an incidence rate of 40-60%. Addition of ATG has shown to reduce its incidence. We report the GVHD outcomes with the use of ATG as part of the prophylaxis regimen in a group of patients (pts) undergoing HSCT.
Methods: From March 2018 to June 2021 we conducted a prospective multicenter study including 95 consecutive pts.: 33 transplanted with an identical sibling donor (ISD), 33 with an HLA-matched unrelated donor(MUD) and 18 with an HLA 1 miss-matched donor (MMUD). Conditioning regimen was according to institutional protocol. GVHD prophylaxis included tacrolimus and methotrexate plus ATG (Timoglobulina®Sanofi) 2.25 mg/kg days -3 and -2.
GVHD incidence, relapse and death were recorded. Acute GVHD (aGvHD) and chronicGVHD (cGVHD) were defined using Glucksberg criteria and NIH clinical grading respectively. Median and interquartile range (IQR) were used to describe non-parametric data, group comparison with X2, Kaplan Meyer curve and multi-sample log Rank test for survival analysis, considering a statistically significant p value of less than 0.05.
Results: 42 pts were male and donors were female in 56% (n:54). Conditioning regimen was myeloablative in 81% (n:77). Median time to neutrophil and platelet engraftment were 17 days (range 15-21) and 17 days (range 12-20) respectively. 6/95 pts presented graft failure.
aGVHD incidence was 36% (n:34), grades I-II 19% (n:19) and III-IV 16% (n:15). Incidence of grades III-IV did not varied according to donor: 15% (n:7) for ISD, 9% (n:3) for MUD and 27% (n:5) for (X2 3.06, p0.21) Overall incidence of cGVHD was 23% MMUD (n:21): Grades mild 6% (n:6), moderate 11% (n:11) and severe 4% (n:4). According to donor type, severe cGVHD was 2% (n:1), 3% (n:1) and 11% (n:2) using ISD, MUD and MMUD respectively (X2 2.65, p0.26). 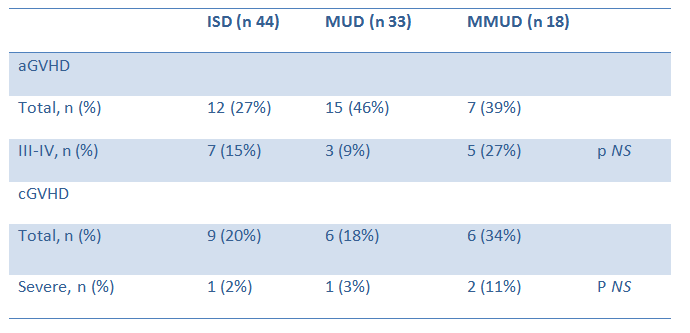
At one year, 15/79 pts (19%) were under immunosuppressive treatment. Transplant related mortality at day 100 was 9% (n:9). Most frequent cause was sepsis (n:4). In 31 pts CMV reactivation was detected (more than one reactivation in 6 pts of which 5 had GVHD) and 7 reactivated EBV infection. None evolved to PTLD.
Median follow-up time was 524 days (IQR 168-833). cGVHD–free and relapse-free survival (combined endpoint) at 1 year was 57% (n:54) of 79 evaluables patients. cGVHD and relapse free survival at 1 year was 48% for IDS (33-63%CI), 66% for MUD (47-81%CI) and 38% for MMUD (21-60%CI). No significant difference was found according to donor type (X2 4.98; p0.083).
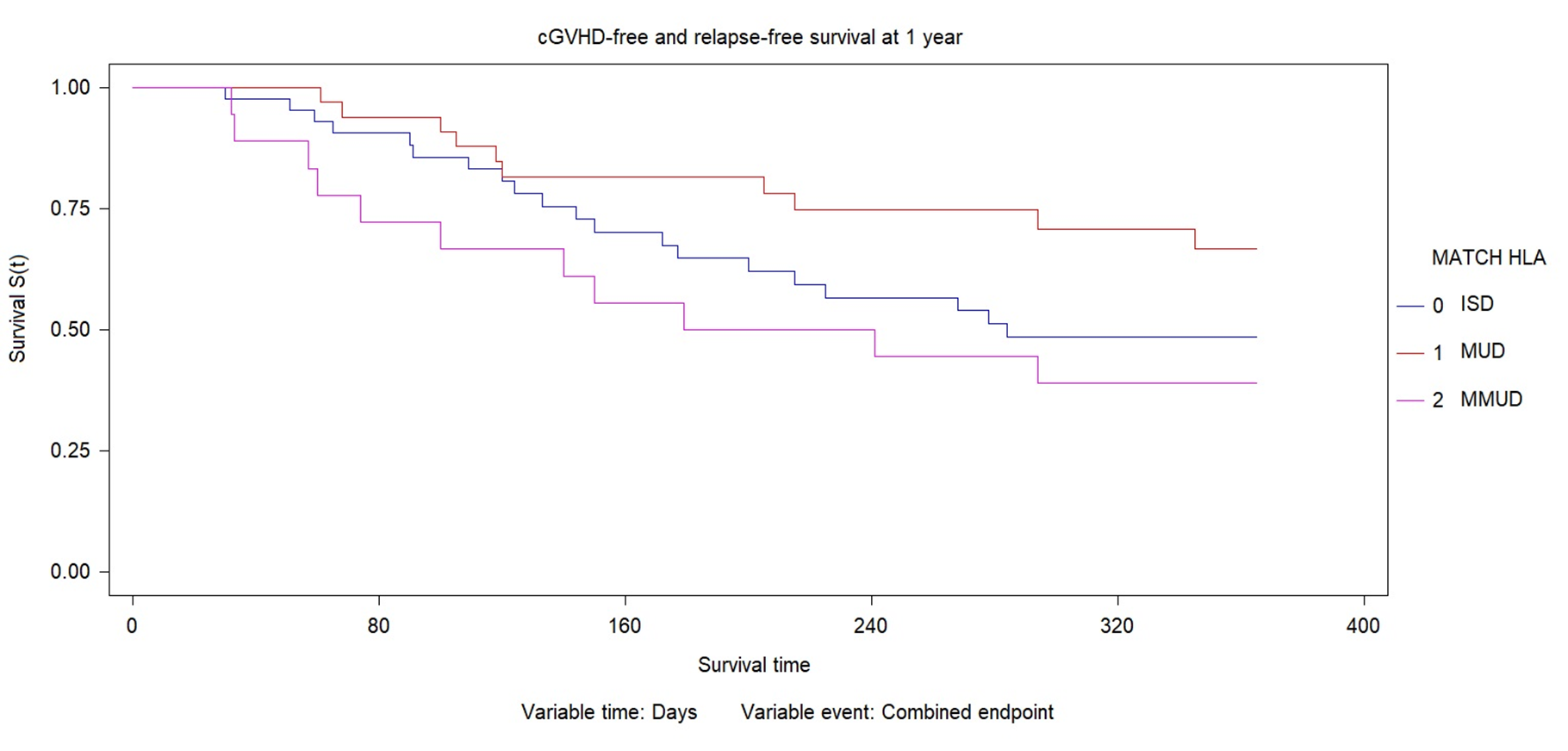
Conclusion: With the use of ATG no difference was observed in the incidence of aGVHD grades III-IV and severe cGvHD between the different types of donor. This results should be confirmed with a higher level of evidence study.

right-click to download
