Availability of using amnion for islet transplantation
Naoaki Sakata1,2, Gumpei Yoshimatsu1,2, Shohta Kodama1,2.
1Department of Regenerative Medicine and Transplantation, Fukuoka University, Fukuoka, Japan; 2Center for Regenerative Medicine, Fukuoka University Hospital, Fukuoka, Japan
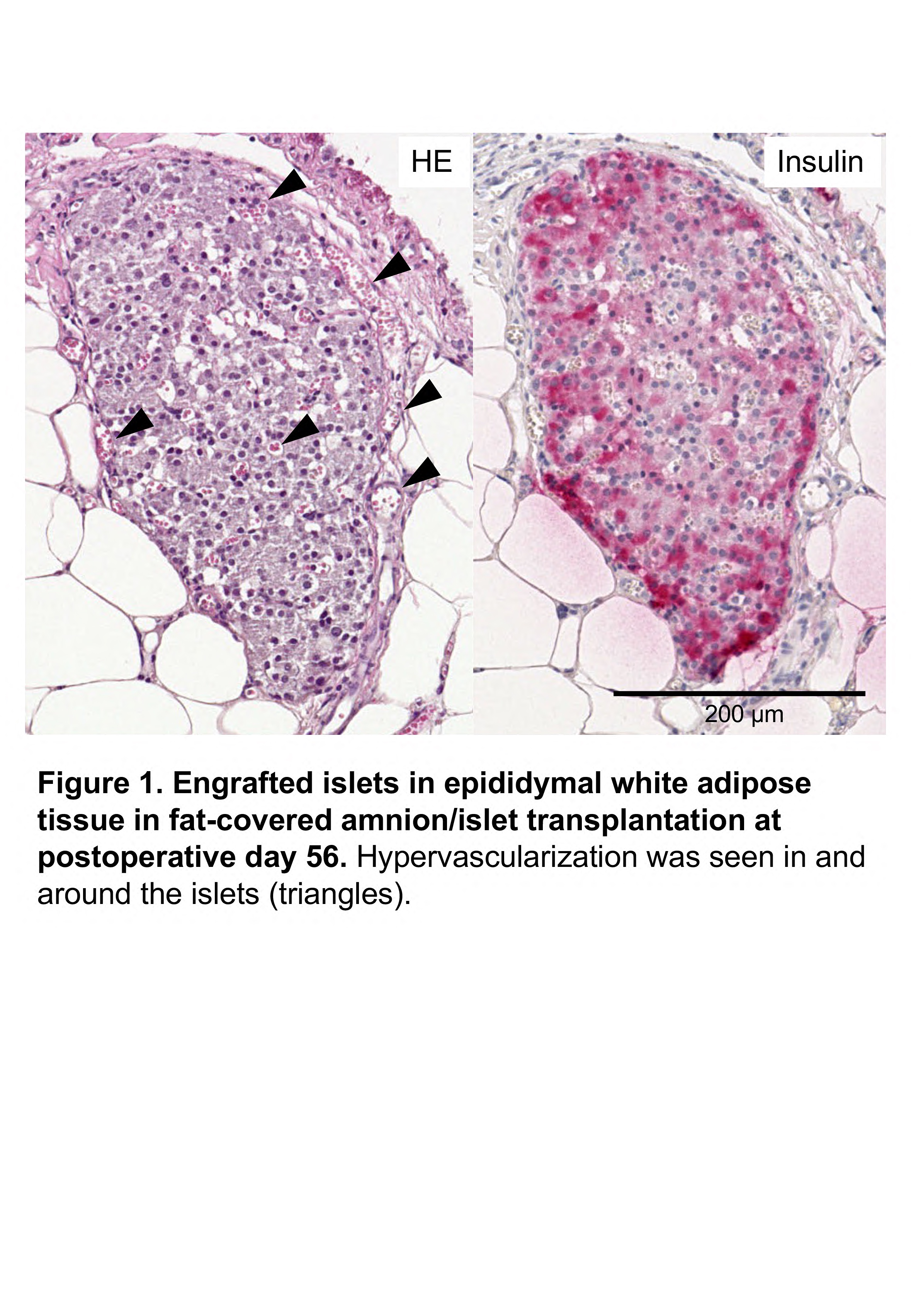
Amnion, a component of the placental membrane which is adjacent to the fetus, is focused on as a promising material for regenerative medicine and transplantation. Amnion includes extracellular matrix, which can be a scaffold for transplanted cells and tissues. Furthermore, amnion contains mesenchymal stem cells. They have multipotencies and play an important role in releasing various growth factors, which promote cellular growth, immune tolerance or angiogenesis. Therefore, it is considered that amnion can be an ideal scaffold for engraftment of transplanted islets. In this study, we attempt to elucidate the usefulness of amnion using animal islet transplantation model. Eight to twelve-week-old C57BL/6J pregnant female mice were used donor of amnion and C57BL/6J male mice as donor of islets and diabetic recipients. Approximately 1.5 cm-sized amnions were used for this study. We tried to assess the effectiveness of amnion by two transplant models. The one was subcutaneous transplant model. Amnion was previously implanted into the axilla of diabetic mouse at 14 days before islet transplantation. 1 cm-sized polyethylene terephthalate tube was inserted into the inner cavity of the amnion as a spacer for transplanted islets. Five hundred islet equivalents (IEQs) were implanted into the inner cavity of amnion after removal of the spacer (amnion-assisted subcutaneous islet transplantation). The other was fat-covered transplant model. Islet (150 IEQs) were infused into inner cavity of amnion and cultured. Then, this amnion containing islets was positioned onto epididymal white adipose tissue of diabetic mice and covered by the tissue (fat-covered amnion/islet transplantation). As a result, two out of four mice received amnion-assisted subcutaneous islet transplantation and one out of four mice received fat-covered amnion/islet transplantation achieved normoglycemia. Regarding later, re-elevation of blood glucose level after graftectomy was confirmed in all four mice received fat-covered amnion/islet transplantation (459.0 ± 87.4 mg/dL vs. 711.0 ± 77.2 mg/dL, p = 0.11). Histologic assessment of recovered fat-covered amnion with islets at postoperative day 56 revealed that engrafted islets with hypervascularization were seen in epididymal white adipose tissue (Figure 1). On the other hand, there was no superiority to islet transplant model without usage of amnion in both subcutaneous and fat-covered transplant models in blood glucose level. Our study showed the possibility of amnion as a scaffold for transplanted Islets, however, islet transplantation using amnion needs further improvement.
Mr. Ryo Kawakami and Mz. Yuriko Hamaguchi for histological examinations.

right-click to download
