Development of an intelligent digital biosurveillance platform
Bartira De Aguiar Roza1, Vanessa Silva e Silva2, Karina Dal Sasso Mendes3, Patricia Treviso4, Tadeu Thome5, Valter Duro Garcia6, Ligia Camera Pierrotti7, Alvaro Machado Dias8, Janine Schirmer1.
1School of Nursing, Federal University of São Paulo, São Paulo, Brazil; 2Department of Nursing, Brock University, St Catherines, Canada; 3Nursing School, São Paulo University of Ribeirão Preto, Ribeirão Preto, Brazil; 4School of Nursing, Vale do Rio dos Sinos University, Porto Alegre, Brazil; 5Transplant Departament, Sírio Libanês Hospital, São Paulo, Brazil; 6Kidney Transplant Department , Santa Casa de Porto Alegre, Porto Alegre, Brazil; 7Division of Infectious and Parasitic Diseases - Clinical Hospital, Faculty of Medicine of the University of São Paulo - FMUSP, São Paulo, Brazil; 8Medicine School, Federal University of São Paulo, São Paulo, Brazil
Organ Donation and Transplantation Research Group - GEDOTT.
This is a study on the development of an intelligent digital platform for Biovigilance, capable of recording structured and unstructured data, in Web and Mobile environments, of sending the automatically compiled data to remote servers, of generating computational panels of analytical results (dashboards) , capable of supporting the production of insights.
The platform (https://biovigilancia.org/home) built for the National Biovigilance System aims to:
(1) Allow the continued recording of new adverse events, in a 100% user-friendly manner. (2) Allow surveys to be carried out with different stakeholders: donors/recipients, hospital managers, health professionals and researchers/teachers. (3) Generate graphs for intuitive visualization of the indicators collected in the surveys, through a results dashboard. (4) Ensure a system of restrictive access so that information of general interest is public, while those with more sensitive characteristics remain accessible only to representatives of the institutions involved in the project.
The platform is divided into three parts (Figure 1):

- Situational research: recurrent mapping of adverse events involving human cells, tissues and organs for transplantation.
- Report an adverse event: register here any adverse events related to human transplants and help Brazilian Biovigilance fulfill its role.
- Restricted functions: this area is intended for researchers and health managers directly involved in the project, with login and password access.
Adverse event reporting is based on the structure of the Notify Library (https://www.notifylibrary.org/) and is designed to allow for broad inclusion and exclusion of variables. Its basic structure separates adverse events as donor-related and recipient-related.
The main restricted functions of the platform are: consultations on sensitive research or not yet publicly disclosed and consultations on the results dashboard involving strategic actions that in some way may be negatively affected by the wide publicity.
The operation of this section came from the implementation of a multi-user authentication system, which allows us to approve specific registrations, blocking subjects without the necessary permissions, as well as cyber attacks.
The National Biovigilance System Platform dashboard is based on a data visualization framework called Shiny. It has graphics of high visual appeal and great informational power. 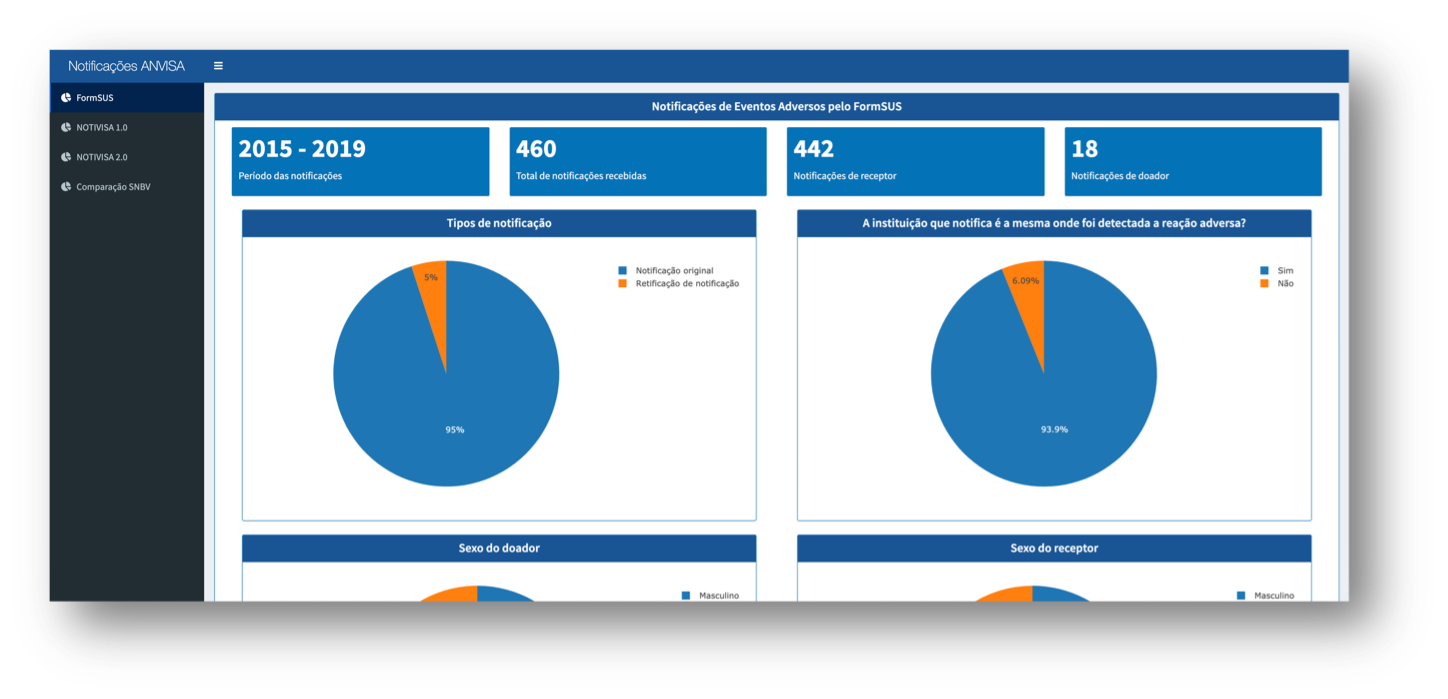
The idea is that the dashboard is continuously fed with collected and reported data, so that the visualizations are always current.
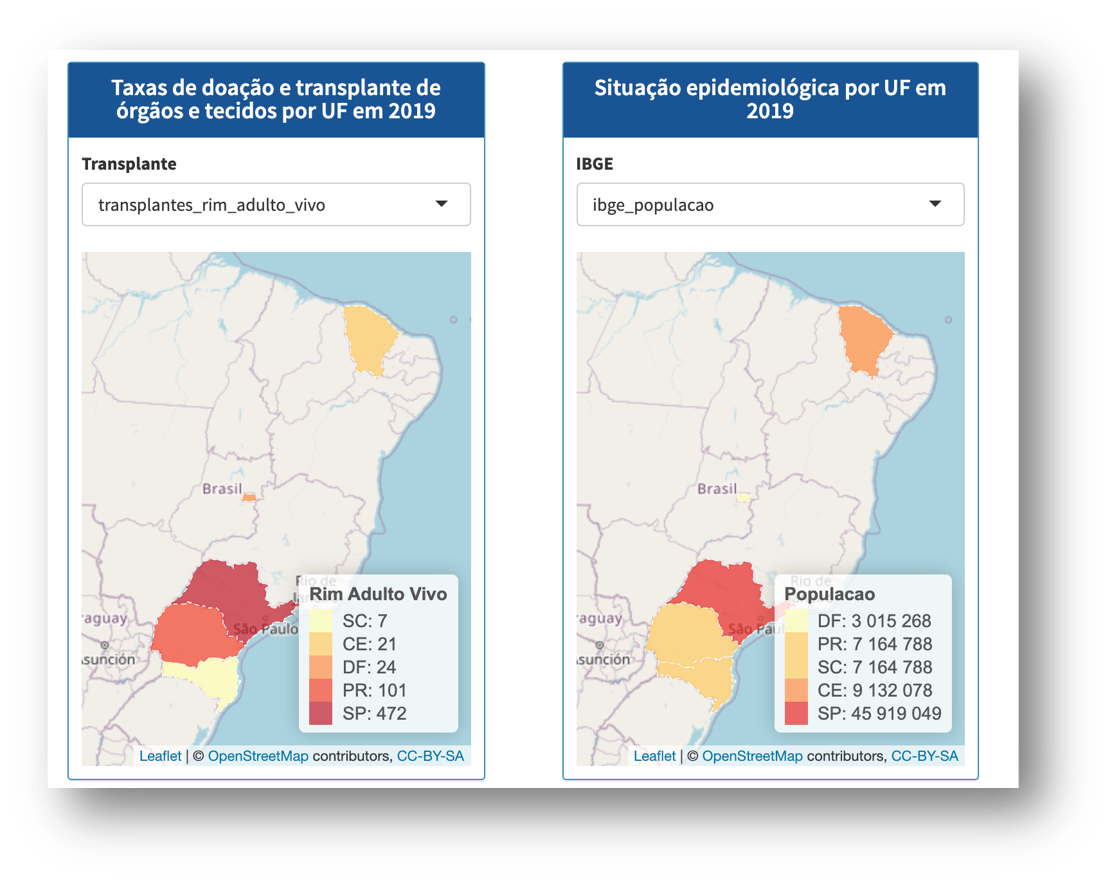
We are currently developing artificial intelligence on the platform so that we can predict the next adverse events and thus contribute to improving safety in the therapeutic use of organs, tissues and cells.
United Nations Development Program - UNDP. National Health Surveillance Agency - ANVISA. Alessandra dos Santos Minervini. Alessandra Duarte Santiago. Arlene Terezinha Cagol Garcia Badoch. Denise de Freitas. Denise Miyuki Kusahara. Eliana Régia Barbosa de Almeida. Érika Bevilaqua Rangel. Heloisa Barboza Paglione. Iara de Oliveira Vitor. Ilka D Fatima Santana Ferreira Boin. João Luis Erbs Pessoa. Joel de Andrade. Letícia de Fatima Lazarini. Maria Cristina Ribeiro de Castro. Maria Helena Costa Amorim. Miguel Angelo de Goes Júnior. Neide da Silva Knihs. Patricia Treviso. Regimar Carla Machado. Renata Fabiana Leite. Samara Ercolin de Souza. Sibele Maria Schuantes Paim. Bruno Henrique Sena Ferreira - Escritório EPE-UNIFESP/BRA/10/008.

right-click to download
