Prasad Gurjar, India has been granted the TTS-ISOT La Renon International Transplantation Science Mentee-Mentor Awards
CYP3A5 polymorphism in renal transplantation: a key to personalized medicine
Prasad Gurjar2, Amit Pasari1,2, Amol Bhawane2, Priyanka Tolani3, Sanjay Kolte4,5, Vijay Katekhaye1, Manish Balwani1,2.
1Department of Nephrology, Saraswati Kidney Care Center, Nagpur, India; 2Department of Nephrology, Jawaharlal Nehru Medical College, Wardha, India; 3Department of Medicine, Jawaharlal Nehru Medical College, Wardha, India; 4Department of Urology, Jawaharlal Nehru Medical College, Wardha, India; 5Department of Urology, Saraswati Kidney Care Center, Nagpur, India
Introduction: Renal transplant (RTp) is the ultimate treatment option for end-stage renal disease (ESRD) patients. Tacrolimus (TAC) is an essential immunosuppressant drug for prevention of rejection in transplant patients. However, TAC has a narrow therapeutic window and its metabolism is affected by the genetic polymorphisms in CYP3A5 gene. TAC dose to maintain the required trough levels may differ according to type of gene polymorphism. In our practice, we observed CYP3A5*3 A6986G polymorphism (GG genotype) as being common finding in most patients undergoing transplant. This prompted us for routine evaluation of CYP3A5 polymorphism for all transplant patients preoperatively. Here, we present the distribution of CYP3A5 genetic polymorphism in evaluated patients and provide TAC dose in different types of polymorphism.
Method: In this retrospective, observational study, the electronic database of our center was screened to identify patients who have been evaluated for genetic polymorphism in CYP3A5 gene. In all patients, genetic analysis was carried via blood sample using polymerase chain reaction (PCR) and restriction fragment length polymorphism (RFLP) method. Data was analyzed descriptively. We considered trough TAC levels of 7 to 10 ng/ml within first six month and 3 to 7 ng/ml after six month of transplant as normal therapeutic levels.
Results: Between January 2017 and 10 March 2022, a total of 31 patients were identified, of which three had wild-type polymorphism. In the remaining 28 (90.0%) cases, CYP3A5*3 A6986G polymorphism was homozygous and heterozygous in 17 (60.7%) and 11 (39.3%), respectively. Among 28 cases, 14 (50.0%) had been diagnosed before transplant. In the 15 who have undergone transplant, 9 (60.0%) and 6 (40.0%) had homozygous and heterozygous polymorphism respectively. Of 15 cases, three did not receive TAC. Table 1 provides the details of dose of TAC required to achieve trough therapeutic levels in first six months and after six months of transplant stratified by type of polymorphism.
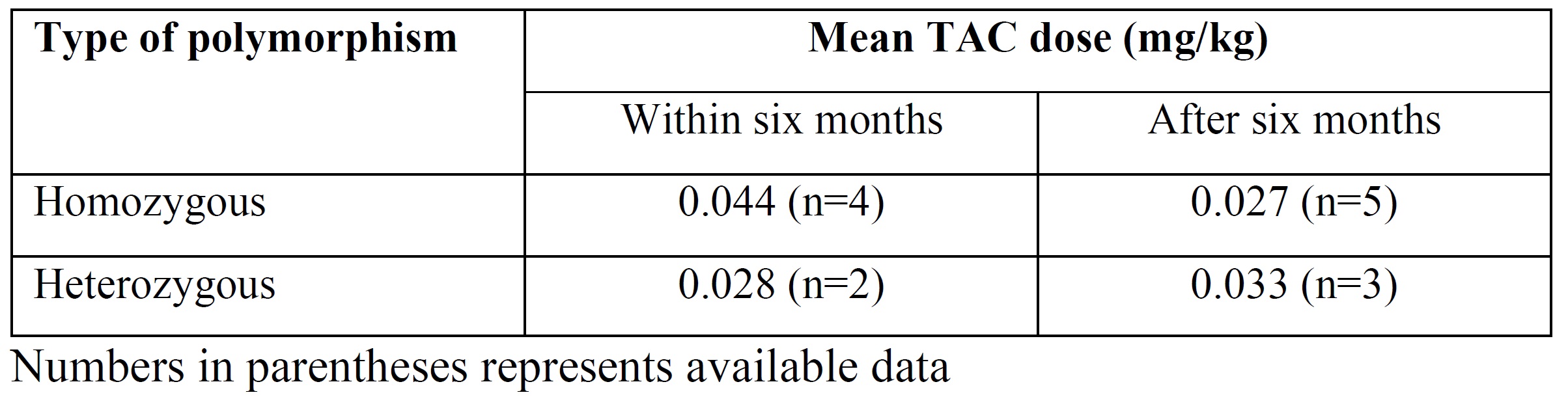
Conclusion: In our geographical area, CYP3A5*3 polymorphism is highly prevalent. We recommend genetic analysis to detect this polymorphism in all planned renal transplant patients. This strategy will help to reduce post-transplant TAC toxicity and will help in achieving personalized immunosuppression.
We acknowledge Ms. Simran Bhanushali and Ms. Nikita Thakre for their contribution in data entry.

right-click to download
