Monotherapy with TNX-1500, a Fc-modified anti-CD154mAb, prolongs cardiac allograft survival in cynomolgus monkeys
Kohei Kinoshita1, Shuhei Miura1,2, Zahra Habibabady1, Franziska Pollok1,3, Madelyn Ma1, Gannon McGrath1, Ryan Chaban1,4, Ivy Rosales5, Siobhan Fogarty6, Patrick Maguire6, Bruce Daugherty6, Seth Lederman6, Richard N Pierson III1.
1Center for Transplantation Sciences, Department of Surgery, Massachusetts General Hospital, Boston, MA, United States; 2Department of Cardiovascular Surgery, Teine Keijinkai Hospital, Sapporo, Hokkaido, Japan; 3Department of Anesthesiology, University Hospital Hamburg-Eppendorf, Hamburg, Germany; 4Department for cardiovascular surgery, University hospital of Mainz, Mainz, Germany; 5Department of Pathology, Massachusetts General Hospital, Boston, MA, United States; 6Tonix Pharmaceuticals, Chatham, NJ, United States
Purpose: Blockade of the CD40/CD154 T cell costimulation pathway is a promising approach to replace conventional clinical immunosuppressive therapy. TNX-1500 (TNX)* is a novel humanized anti-CD154 mAb that contains the hu5c8 Fab region and an Fc region engineered to modulate FcγR2 binding to reduce the risk of thromboembolic events seen with hu5c8 IgG1 in previous clinical trials. Its efficacy was evaluated in cynomolgus monkey heterotopic heart allograft recipients.
Methods: Cynomolgus monkey heterotopic cardiac allograft recipients were treated with TNX 30mg/kg iv 2x weekly for 2 weeks, then either a ‘standard-dose’ regimen of 20mg/kg weekly from days 21-180 (sTNX, n=5) or a reduced-dose TNX maintenance regimen (10 mg/kg weekly x6, then 20mg/kg monthly; loTNX), with (loTNX+MMF, n=4) or without mycophenolate mofetil (MMF) at 200mg/d po (loTNX, n=4). In 3 sTNX animals therapy was discontinued on day 175. Results were compared to historical data with hu5c8 monotherapy dosed as for loTNX (n=5). Biopsies or explanted hearts were evaluated at three protocol-defined time-points (d45, d90, d180), at earlier explant.
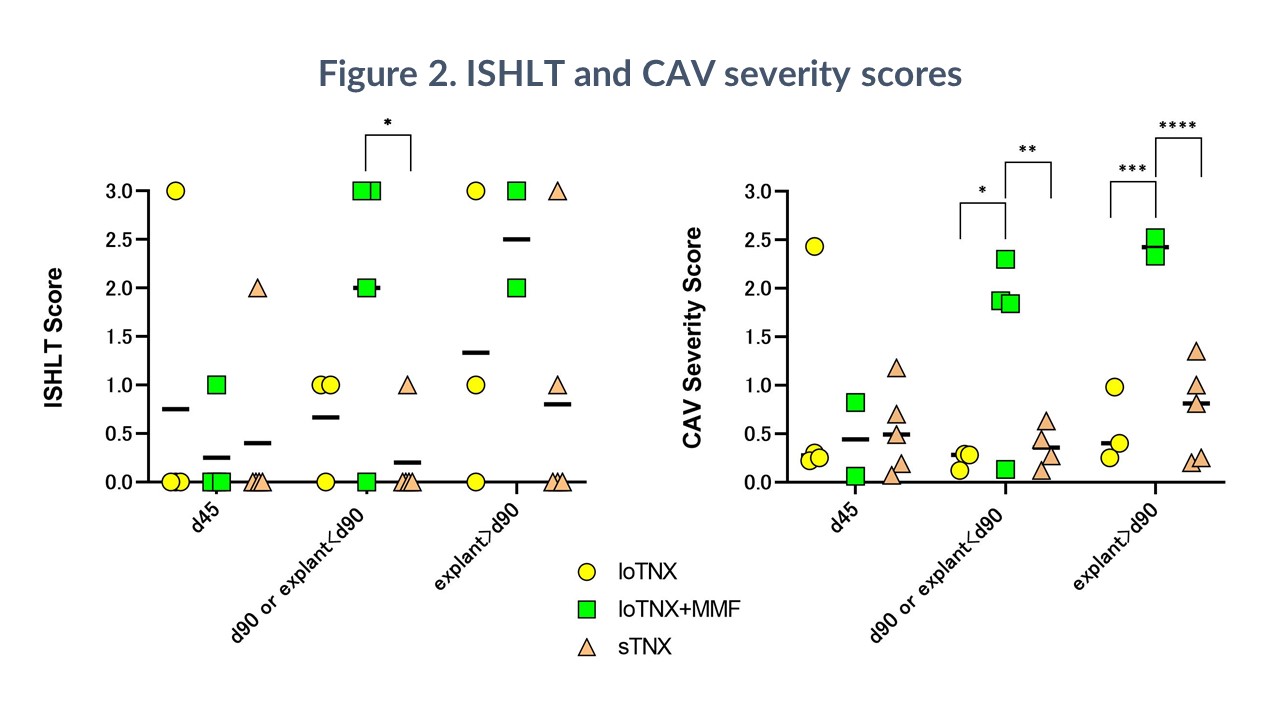
Results: sTNX (MST >232d, range 116-305) significantly prolonged cardiac allograft survival relative to hu5c8 alone (MST 133d; p=0.016), loTNX (MST 99d; p=0.014), or loTNX+MMF (MST 88d; p=0.011). All grafts treated with loTNX () or with loTNX+MMF () exhibited acute rejection histologically within 90 days, and 7 of 8 grafts failed before day 180. One sTNX graft rejected at 116d in association with bacterial infection; one graft was electively explanted at 181d; and three grafts survived for 57, 90, and 130 days after discontinuing TNX-1500. Neither sustained thrombocytopenia nor micro- or macro-vascular thrombosis were observed. At 90 days, loTNX+MMF had a significantly higher median ISHLT score (2.0) than sTNX grafts (0.2; p=0.016); loTNX+MMF also had a significantly higher median CAV score (1.8) at d90 than either loTNX (0.2; p=0.022) or sTNX (0.3; p=0.026). At subsequent graft explant or end-of-study biopsy (180d), loTNX+MMF had a higher median CAV score (2.4) than loTNX (0.4; p=0.002) or sTNX (0.8; p=0.002). sTNX suppressed the rise in Teff/Tregs ratio at the final time-point (180d or <180d if graft failure) more effectively than loTNX+MMF (3.5 ± 0.9 vs 9.5 ± 6.1, p=0.031). sTNX (5/5) but not loTNX (1/4) or loTNX+MMF (2/4) consistently prevented anti-donor antibody elaboration.
Conclusions: Blockade of CD154 with sTNX monotherapy was not associated with thrombotic events, well tolerated, and consistently prevented pathogenic alloimmunity in this stringent preclinical model. Lower TNX maintenance dosing, with or without MMF, was less effective.
*TNX-1500 is a pre-IND molecule and not approved for any clinical indication.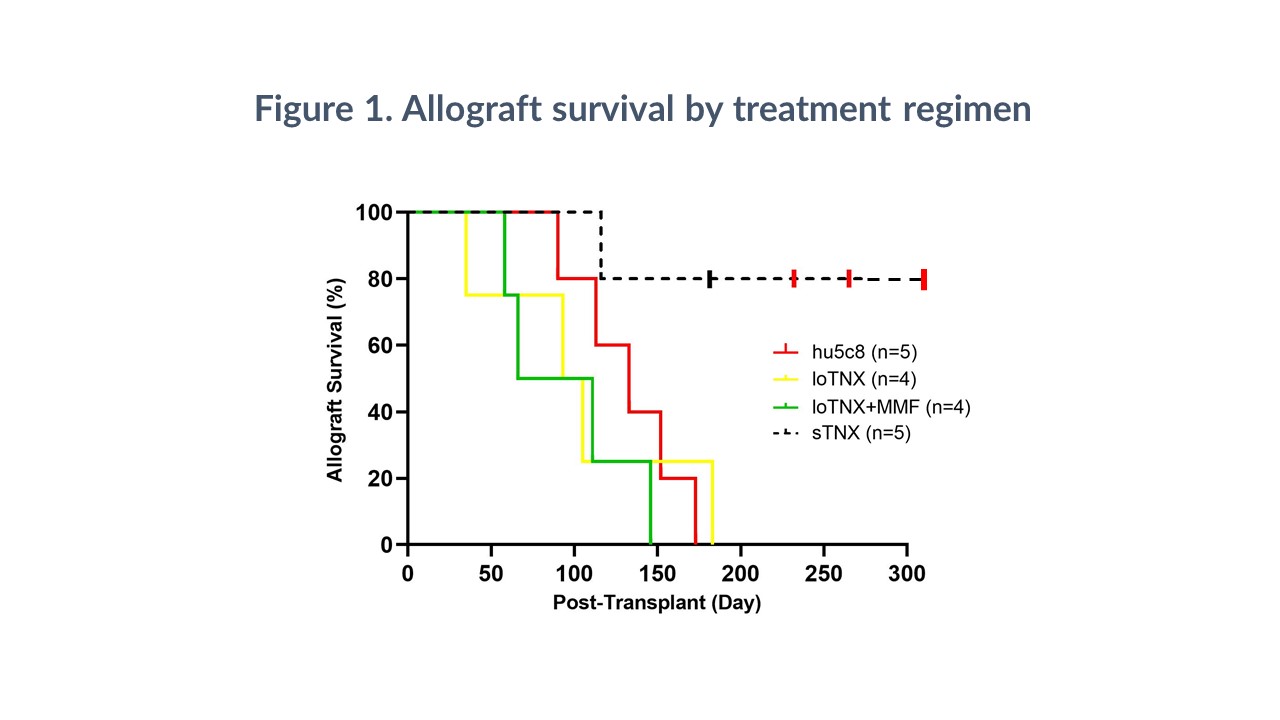
NIH grant P01 HL158504. Tonix Pharmaceuticals. RC is supported by the Benjamin Research Fellowship from the German Research Foundation.

right-click to download
